Abstract
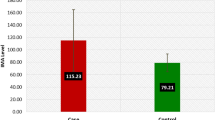
Preeclampsia is a multisystem disorder involves altered homeostasis of oxidants–antioxidants, inflammatory process and endothelial dysfunction. The present study aim was to determine the levels of oxidative stress parameters (malondialdehyde, protein carbonyl, ischemia modified albumin and xanthine oxidase), nutrient antioxidants (vitamin C and vitamin E), enzyme antioxidants (catalase, superoxide dismutase, glutathione peroxidase glutathione reductase), total antioxidant status (TAS) and its association with nitric oxide. The study population consists of three groups, non pregnants (Group 1, n = 57), normotensive pregnants (Group 2, n = 57) and Preeclampsia (Group 3, n = 57). Group 2 and 3 were followed after delivery within 48 h. In preeclampsia xanthine oxidase, malondialdehyde and uric acid levels were significantly increased (p < 0.001), while TAS decreased (p < 0.05) when compared to normotensive pregnant and non pregnant. Catalase, glutathione reductase levels were increased (p < 0.005) and vitamin E, super oxide dismutase levels were decreased (p < 0.001) in preeclampsia when compared to normal pregnants. Receiver operating characteristics curve analysis showed area under curve for xanthine oxidase (0.8), malondialdehyde (0.804), Uric acid (0.84), ischemia modified albumin (0.92) and catalase (0.88) which indicated as good markers in preeclampsia. Amongst, ischemia modified albumin is a better marker of intrauterine hypoxic reperfusion risk with sensitivity 87.7 % and specificity 91.2 %. The increased hydrogen peroxide from xanthine oxidase adds to oxidative stress and increased catalase activity in preeclampsia represents combating action. Increased oxidative stress, decreased TAS and its apparent reversible changes evinced within 48 h after delivery in preeclampsia illustrated that placental abnormality is the contributing factor in the pathogenesis.
Similar content being viewed by others
References
Sibai BM. Hypertensive disorders of pregnancy: the United States prospective. Curr Opin Obstet Gynecol. 2008;20:102–6.
Betran AP, Wojdyla D, Posner SF, Gülmezoglu AM. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health. 2005;5:131.
Kurlak LO, Green A, Loughna P, Broughton Pipkin F. Oxidative stress markers in hypertensive states of pregnancy: preterm and term disease. Front Physiol. 2014. doi:10.3389/fphys.2014.00310.
Bilodeau JF. Review: maternal and placental antioxidant response to preeclampsia—impact on vasoactive eicosanoids. Placenta. 2014;35:S32–8.
Karacay O, SepiciDincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, et al. A quantitative evaluation of total antioxidant status andoxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res Clin Pract. 2010;89(3):231–8.
Bambrana V, Dayanand CD, Kotur PP. Is xanthine oxidase, a marker in pre-eclampsia? A case–control study. J Clin Diagn Res. 2015;9(10):BC 1–3.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Bergmeyer HU, Gawehn K, Grassl M. Glutathione reductase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. p. 465–6.
Bar Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—a preliminary report. J Emerg Med. 2000;19(4):311–5.
Trivedi RC, Rebar L, Berta E, Stong L. New enzymatic method for serum uric acid at 500 nm. Clin Chem. 1978;24(11):1908–11.
Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6.
Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–2.
Mavis RD, Stellwagen E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J Biol Chem. 1968;243(4):809–14.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
Roe JH. Comparative analyses for ascorbic acid by the 2, 4-dinitrophenylhydrazine method with the coupling reaction at different temperatures: a procedure for determining specificity. J Biol Chem. 1961;236:1611–3.
Martinek RG. Method for the determination of vitamin E (total tocopherols) in serum. Clin Chem. 1964;10:1078–86.
Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174:288–91.
Murata M, Fukushima K, Takao T, Seki H, Takeda S, Wake N. Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J Reprod Dev. 2013;59(1):7–13.
Kaur G, Mishra S, Sehgal A, Prasad R. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol Cell Biochem. 2008;313:37–44.
Zusterzeel PL, Mulder TP, Peters WH, Wiseman SA, Steegers EA. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radic Res. 2000;33(5):471–6.
Bakacak M, Kılınç M, Serin S, Ercan Ö, Köstü B, Avcı F, et al. Changes in copper, zinc, and malondialdehyde levels and superoxide dismutase activities in pre-eclamptic pregnancies. Med Sci Monit. 2015;21:2414–20.
Khera A, Vanderlelie JJ, Perkins AV. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta. 2013;34(7):594–8.
Suhail M, FaizulSuhail M, Khan H. Role of vitamins C and e in regulating antioxidant and pro-oxidant markers in preeclampsia. J Clin Biochem Nutr. 2008;43(3):210–20.
Ikpen MA, Eigbefoh J, Eifediyi RA, Isabu PA, Okogbenin S, Okogbo FO, et al. Determination of antioxidant status of pre-eclamptic and normotensive sub-rural Nigerian pregnant women at the Irrua Specialist Teaching Hospital, Irrua, Edo State. J Matern Fetal Neonatal Med. 2012;25(10):2046–50.
Papageorghiou AT, Prefumo F, Leslie K, Gaze DC, Collinson PO. ThilaganathanB. Defective endovascular trophoblast invasion in the first trimester is associated with increased maternal serum ischemia-modified albumin. Hum Reprod. 2008;23(4):803–6.
Soto ME, Soria-Castro E, Lans VG, Ontiveros EM, Mejia BI, Hernandez HJ, et al. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid Med Cell Longev. 2014;. doi:10.1155/2014/760694.
Kenet G, Freedman J, Shenkman B, Regina E, Brok-Simoni F, Holzman F, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol. 1999;19(8):2017–23.
Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63(3):976–81.
Vanderlelie J, Gude N, Perkins AV. Antioxidant gene expression in preeclamptic placentae: a preliminary investigation. Placenta. 2008;29(6):519–22.
Acknowledgments
We would like to thank the authorities of Sri Devaraj Urs Academy of Higher Education and Research for supporting this doctoral study.
Funding
Self-funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mrs. Vanishree Bambrana, Dr. C. D. Dayanand and Dr. Pushpa Kotur declare that they have no conflict of interest. Financial support for this work borne by authors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Research Involving Human Participants and/or Animals
This is non interventional study. Sample collection from human participants under taken after obtaining Institutional Ethical Committee approval. An appropriate individual patient informed consent used for sample collection. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bambrana, V., Dayanand, C.D. & Kotur, P. Relationship Between Xanthine Oxidase, Ischemia Modified Albumin, Nitric Oxide with Antioxidants in Non Pregnants, Pre and Post-delivery of Normal Pregnants and Preeclampsia. Ind J Clin Biochem 32, 171–178 (2017). https://doi.org/10.1007/s12291-016-0599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0599-0