Abstract
Purpose
Opioids have a narrow therapeutic index and have the potential to cause significant harm. Developmental and pharmacogenetic factors put children, and especially infants, at increased risk of complications. We performed a retrospective root cause analysis to identify the factors associated with critical incidents in children receiving opioid infusions in a tertiary care children’s hospital.
Methods
Following institutional ethical approval, we identified potential critical incidents during 2004 to 2009 from patient safety and pharmacy data. Patients’ medical charts were reviewed and a timeline of events that occurred before, during, and following each incident was generated. A safety assessment code score was assigned to each incident according to its severity and probability of recurrence, and incidents with a score ≥ 8 were selected for root cause analysis. Root causes were identified and classified, formal causal statements were written, and action plans were recommended.
Results
One hundred and sixty-six medical charts were reviewed, and 58 of these included one (45/58) or more (13/58) relevant critical incidents. The resulting harms were of minor to moderate severity. Fourteen incidents were submitted for detailed analysis, from which 31 root causes were identified. The most frequent and significant root causes involved defects in pre-printed order sheets, lack of a nursing guidelines for infusions (rate, adjustment, weaning), and inadequate guidelines for monitoring and recording pain, vital signs, and arousal scores.
Discussion
The root causes of a range of critical incidents have been identified, and these have been used to generate recommendations for improving both patient safety and quality of analgesia for children receiving opioid infusions for acute pain management.
Résumé
Objectif
Les opioïdes ont un indice thérapeutique étroit et peuvent avoir des conséquences graves. En raison de facteurs développementaux et pharmacogénétiques, les enfants, et tout particulièrement les nourrissons, courent un risque accru de complications. Nous avons réalisé une analyse rétrospective des causes fondamentales afin d’identifier les facteurs associés aux incidents graves chez les enfants recevant des perfusions d’opioïdes dans un hôpital pédiatrique de soins tertiaires.
Méthode
Après avoir obtenu le consentement du comité d’éthique de notre institution, nous avons identifié les incidents potentiellement graves survenus entre 2004 et 2009 en analysant les données de sécurité et de pharmacie des patients. Les dossiers médicaux des patients ont été passés en revue et un calendrier des événements survenus avant, pendant et après chaque incident a été généré. Une note de code d’évaluation d’innocuité a été attribuée à chaque incident selon sa gravité et la probabilité de sa récurrence, et les incidents avec une note ≥ 8 ont été retenus pour analyse des causes fondamentales. Les causes fondamentales ont été identifiées et classifiées, des énoncés causaux formels ont été rédigés, et des plans d’action recommandés.
Résultats
Cent soixante-six dossiers médicaux ont été passés en revue, dont 58 rapportaient un (45/58) ou plusieurs (13/58) incidents graves pertinents. Les lésions qui en ont résulté étaient de gravité faible à modérée. Quatorze incidents ont été soumis à une analyse détaillée, et 31 causes fondamentales ont été identifiées. Les incidents les plus fréquents et importants impliquaient des défauts dans les feuilles d’ordonnance pré-imprimées, un manque de directives aux infirmières concernant les perfusions (vitesse, ajustement, sevrage), et des directives inadaptées pour le monitorage de la douleur, des signes vitaux et des scores d’éveil, ainsi que pour la transcription de ces données.
Discussion
Les causes fondamentales de toutes sortes d’incidents graves ont été identifiées, et elles ont été utilisées pour formuler des recommandations permettant d’améliorer la sécurité des patients et la qualité de l’analgésie des enfants recevant des perfusions d’opioïdes pour soulager la douleur aiguë.
Similar content being viewed by others
A critical incident is an event or situation that creates a significant risk of serious harm to the physical or mental well-being of a patient.1 An adverse drug reaction (ADR) involves harm that is directly caused by a drug administered at normal doses.2 Opioid analgesics are amongst the most commonly administered pharmacological agents used to control moderate to severe nociceptive pain in children.3-5 Nevertheless, these agents are also among the most dangerous drugs prescribed in the ward setting with the potential to cause ADRs and/or harm resulting from the occurrence of a critical incident.6-9
Opioids may be administered by epidural infusion or intravenously via continuous opioid infusion (COI) or patient-controlled analgesia (PCA). Critical incidents related to the use of opioid infusions have the potential to cause severe harm such as neurologic injury due to hypoxemia, cardiorespiratory dysfunction/failure, or death.7-10 The most common ADRs associated with opioids are nausea/vomiting (42.5%), urinary retention (13.5%), pruritus (12.7%), oversedation (0.9%), dysphoria, respiratory depression (0-7%), and constipation.11,12 In addition, 65% of patients receiving COI report inadequate analgesia,12 which in children is associated with increased postoperative morbidity and mortality.13-15 While intermittent doses of oral or systemic opioids are not without significant safety issues, opioid infusions carry a broader set of safety concerns, including the need for ongoing monitoring without the continuous presence of a trained observer, and have defined the scope of an extensive audit conducted by the Association of Paediatric Anaesthetists of Great Britain and Ireland.7
Risks to patient safety are influenced by population-specific factors.16 Children, especially infants, represent a high-risk group for opioid-related critical incidents because of developmental and pharmacogenetic differences in their pharmacological response to opioids. In addition, pediatric opioid pharmacokinetic data are lacking for many commonly used drugs, so dosing recommendations are often empirical and based on adult data.17-19 Opioids at these scaled-down doses for children are considered safe, but evidence of efficacy and safety in the pediatric population is limited.20
Root cause analysis (RCA) is an established methodology for critical incident analysis.21-23 In 2006, the Canadian Patient Safety Institute (CPSI) defined RCA as “an analytic tool that can be used to perform a comprehensive, system-based review of critical incidents. The goals of RCA are to determine what happened, why it happened, and what can be done to reduce the likelihood of a recurrence. It includes the identification of the root and contributory factors, determination of risk reduction strategies, and development of action plans along with measurement strategies to evaluate the effectiveness of the plans.”22 The CPSI’s revised 2012 incident analysis framework continues to recommend the use of RCA techniques in an expanded methodology.23
Using this RCA methodology, we conducted a five-year retrospective audit to examine critical incidents related to the use of opioids administered intravenously or via the epidural route that occurred at BC Children’s Hospital (BCCH) during December 2004 to December 2009. The aim of this audit was to use the collected information to make recommendations to support changes in practice in order to improve the safety and quality of analgesia for children receiving opioid infusions in the future. A secondary aim was to determine if involvement of the acute pain service (APS) team in a child’s care had any influence on these critical incidents.
Methods
Ethical approval was obtained from the University of British Columbia Children’s and Women’s Research Ethics Board to conduct a retrospective review of cases spanning December 2004 to December 2009. This time frame is consistent with a recent study8 and was deemed likely to yield analysis of actions that may still exist in current policies, procedures, and practices. The review was conducted at BCCH from January 2010 to December 2011. Lists of potential critical incidents in children receiving opioid infusions were obtained from the Patient Safety and Learning System (PSLS) and from the Pharmacy Department. The PSLS is a voluntary incident reporting database that was searched using keywords to identify potential incidents occurring in all relevant areas of the hospital. Information from the Pharmacy Department included a list of children for whom opioids were prescribed during the study period and a list of naloxone withdrawals from the pharmacy system, which indicates possible naloxone administration and a potential opioid-related incident.
Medical charts relating to the potential incidents were assessed for inclusion in the study according to the following criteria: (a) the patient was receiving a continuous intravenous or epidural opioid infusion (patients receiving bolus doses of opioids were excluded); (b) the opioid infusion was prescribed for acute pain management; (c) sufficient evidence existed for the occurrence of a critical incident related to the opioid infusion; (d) sufficient information existed regarding the related events in the patient’s medical chart and/or the PSLS report to allow a reasonable analysis of the incident. If appropriate, the APS database was reviewed for further information.
Demographic data, body weight, patient comorbidities, opioid indication, analgesic orders (including methods, drugs, doses, and routes), administration details (including the prescribing and attending physician’s specialty and grade) were recorded on a datasheet. All study materials and datasheets were locked in a secure location, and data were summarized in password-protected Excel spreadsheets (Microsoft, Redmond, WA, USA).
The charts were evaluated in depth to determine the nature of the critical incidents, including the place and time of occurrence, the reporting person, and the type of monitoring before and at the time of the incident. A timeline of events that occurred before, during, and following the incident was generated. Critical incidents were categorized by type of harm, type of analgesia, and the service caring for the patient.
It is institutional practice at BCCH that the APS directly supervise all children prescribed PCA, epidural analgesia, COI in infants less than three months of age, and COI utilizing hydromorphone or fentanyl. The responsibility for pain management for most cases of COI utilizing morphine rests with the primary surgical or medical specialty without involvement from the APS team. The APS is consulted as required, for example, following evidence of inadequate analgesia.
Harm was deemed to have occurred when interventions were performed that would not normally be required. Types of critical incidents included patients requiring bag-valve-mask ventilation, unexpected tracheal intubation, cardiac arrest, death, prolonged hospital stay, acquired neurologic deficit, unexpected admission to the pediatric intensive care unit (PICU), inadequate analgesia, naloxone administration, and code blue call (emergency call for cardiorespiratory dysfunction or failure).
The incidents were classified according to a safety assessment code (SAC) whereby the incidents are scored by their severity and the probability of their recurrence, and these two numbers were multiplied to determine the final coding (Table 1).24,25 Incidents with SAC scores of ≥ 82,22 were selected for RCA, which was conducted by the authors in consultation with the BCCH Patient Safety, Quality & Accreditation leader and a clinical nurse educator.
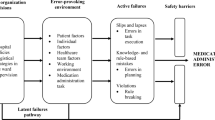
A reality tree diagram was used to map the factors contributing to a critical incident22 with the aim of establishing how and why the incident occurred by identifying active and latent failures in the sequence of events. A reality tree diagram was drawn for each incident using Visio 2003 software (Microsoft, Redmond, WA, USA). Considering current and previous policies and procedures, a series of “caused by” and “why” questions were used to find the causal and contributing factors for each incident using pre-defined triage and triggering questions as prompts. The identified root causes were classified according to these conceptual categories for triggering questions: communication, training, fatigue/scheduling, environment/equipment, rules/policies/procedure, or barrier.22,23 Formal causal statements were written according to the following guideline: a set of circumstances (A=Antecedent) increased the likelihood (B=Behaviour) that this set of consequences (C=Consequences) would occur.22 Based on these causal statements, action plans were recommended to eliminate or control the root causes and thereby prevent the incidents from recurring.
Results
Denominator data
During the study period, the APS managed 906 patients on PCAs, 479 patients on epidural infusions, and 389 patients receiving other pain management techniques, including COI. The BCCH Pharmacy Department processed orders for 2,086 COIs. These denominator data are not exact, however, as not all COI infusions for non-APS patients were prescribed using the standard order set during the period under review; hence, the total number of COIs administered is unknown.
Initial case review
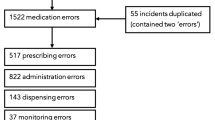
Based on potential incidents identified from the PSLS and the Pharmacy Department naloxone data, 166 medical charts were assessed for inclusion in the study (Figure). Fifty-eight of these (49 from the PSLS list and nine from the Pharmacy Department list) contained evidence of one or more relevant critical incidents. Patient characteristics are presented in Table 2. The critical incidents identified included opioid administration error (n = 39/58, 67%), prescription error (including prescription of concurrent opioids) (n = 18/58, 31%), infusion pump malfunction (n = 4/58, 7%), and respiratory depression with or without naloxone administration (n = 6/58, 10%). More than one critical incident occurred in 13/58 (22%) cases.
Administration errors included pump programming errors in which the infusion rate, concentration, or dose of drug was set incorrectly (n = 13/58, 22%), errors in selecting or mixing the prescribed drug concentration (n = 7/58, 12%), and drug labelling errors (n = 5/58, 9%). One case involved administration of the wrong drug (midazolam instead of morphine), and in 4/58 (7%) cases, a drug was given at the wrong time (in three of these, an oral opioid was given in error either at the same time as or immediately after discontinuing an opioid infusion). Problems with setup and maintenance of intravenous access occurred in 5/58 (9%) cases.
In 11/58 (19%) incidents, the error was identified and corrected before any drug was administered; in these “near miss” cases, the error was in the prescription, the drug labelling, and/or the location of drugs. The incidents reviewed included 8/58 (14%) instances of inadequate analgesia and one emergency cardiorespiratory call (“code blue”). No other serious harm was experienced; that is, there were no instances of unexpected PICU admission, bag-valve-mask ventilation, unexpected intubation, prolonged hospital stay, cardiac arrest, acquired neurologic deficit, or death.
At the time the critical incident occurred, 40/58 (69%) patients were receiving COI morphine, 3/58 (5%) were receiving COI hydromorphone, 1/58 (2%) was receiving COI fentanyl, 7/58 (12%) had a PCA, and 2/58 (3%) were on an epidural infusion. Of the remaining cases, 2/58 (3%) had been transferred from an intravenous opioid infusion to PO codeine by the time of the incident, 2/58 (3%) were receiving a combination of COI and oral morphine, and 1/58 (2%) was prescribed COI morphine by the APS and intravenous morphine PRN by the surgical team.
Root cause analysis
Sixteen of the 58 incidents reviewed were given a SAC score of ≥ 8 and were selected for RCA. Insufficient data were available in health records for two of the selected incidents. Root cause analysis was performed by the study team on 13 cases, and results for the remaining incident were taken from a RCA conducted by the institution (Table 3).
In five of the RCA cases, some gaps were identified in SpO2, respiratory rate, pain score, sedation score, and intravenous status recordings. In one of the RCA cases, hourly infused amounts of morphine were not documented. Respiratory depression and/or oversedation occurred in six of the RCA cases; in four of these cases, naloxone was administered. Both prescription errors and administration errors were identified in these incidents. Two incidents of respiratory depression, lacking proper documentation of pain and arousal scores, occurred in the PICU in children under one week of age. Another two of these incidents occurred in children receiving regular doses of oral codeine within 24 hr of discontinuing an opioid infusion. Four of these six incidents of respiratory depression occurred between 3:00 am and 7:00 am.
From the RCA of these 14 incidents, 31 distinct root causes were identified and classified. The root causes identified were classified as communication (4/31), training (5/31), environment/equipment (5/31), or rules/policies/procedure (17/31). Although the effectiveness of barriers was included in the analysis, none of the root causes were given this as a primary classification; insufficient data were available to identify fatigue/scheduling causes. Each root cause was used to generate an organizational recommendation, some of which have already been implemented. These root cause classifications, recommendations, and actions taken are summarized in Table 4.
Critical incidents in APS and non-APS patients
Lack of definitive denominator data for non-APS patients prevents a reliable comparison with APS patients. Of the 58 critical incidents reviewed, 25/58 (57%) occurred in patients who were currently under the care of the APS; this represents an incident rate of 1.4% among APS patients. In five of these cases, patients had been receiving COI morphine under the care of non-APS services and were subsequently referred to the APS. Of the 14 RCA cases, the APS were not involved in the care of 9/14 (64%) patients and had only partial involvement in the care of four (29%) others.
Discussion
In this retrospective five-year review, we identified 58 critical incidents from a total of more than 3,500 administered opioid infusions, equivalent to an overall incident rate of 1.7%. None of these incidents resulted in significant harm to a child. The majority (39/58, 67%) of the incidents identified involved some error during drug administration; prescription errors were identified in 18/58 (31%) incidents and pump errors in 4/58 (7%) incidents. Fourteen cases, selected on the basis of severity and likelihood of recurrence, were submitted to in-depth RCA. This analysis revealed 31 root causes from which recommendations were derived for improvements in the following aspects of service delivery: communication, training, environment/equipment, and rules/policies/procedure.
In 2009, the Institute for Safe Medication Practices Canada identified morphine as the drug most frequently reported for causing harm through medication error.6 In the United Kingdom, a prospective audit of 10,000 pediatric patients receiving opioid infusions managed by an Acute Pain Team showed an incidence of 0.42% of serious clinical incidents.7 A comprehensive review of 5,935 voluntary medication-related safety reports in a tertiary university-affiliated children’s hospital in Ontario, Canada confirmed that opioids are the most common agent implicated in drug error leading to patient harm, that morphine was the most frequently reported opioid drug of error, and that administration was the most frequently reported stage of medication error.8 A recent review of case reports identifies some specific patterns in clinical conditions in the development of opioid-induced respiratory depression in children 12 yr of age or younger. However, the review also points out that eight of the 24 (33%) reports reviewed describe errors in drug administration.9 The Canadian Paediatric Adverse Events Study suggests that nearly half (44.7%) of all adverse events are preventable.26
During the period under review in this study, standard opioid prescription orders and nursing protocols did exist at BCCH in an attempt to ensure safe care of children receiving epidural, COI, and PCA opioids. Workshops and assessments were held regularly for nurses managing pediatric acute pain. These were supported by regularly updated online nursing policies and procedures for opioid administration. Nonetheless, despite these procedures, critical incidents occurred. The reasons for this are multifactorial, including: inadequate monitoring (involving an emphasis of nursing protocols on SpO2 monitoring, which is an unreliable monitor of respiratory depression when patients receive supplemental oxygen);27 staff expertise; equipment malfunction; conversion errors between different opioid protocols; and/or communication breakdowns when children were transferred between different areas of the institution (e.g., the PICU, which used different opioid concentrations and policies).
Twelve (39%) of the 31 root causes identified in this review have been addressed in a recent hospital-wide implementation of standard opioid concentrations, which included revised pre-printed orders and nursing policies. In early 2012, BCCH agreed to adopt standard concentrations throughout the institution, including critical care areas such as the PICU. All systemically infused opioids (COIs and PCAs) are now prescribed with standard institutional pre-printed orders containing updated nursing policies and procedures to support their use. The Pyxis® dispensing system (CareFusion, San Diego, CA, USA) protects against withdrawal of a wrong opioid concentration, and systemic opioids are infused using Alaris® pumps (CareFusion, San Diego, CA, USA) with maximum infusion limits and weight-based checking using patient-identified bar-coded syringes with contents/opioid concentration made up exclusively in the Pharmacy Department. Nursing policies now include more specific guidelines for infusion rate adjustment and for arousal monitoring, especially in infants and both before and after the administration of opioid bolus doses with concurrent opioid infusions. Monitoring was improved with new flow sheets requiring an increased level of documentation of pain-related variables, such as opioid usage each hour, pain scores, and vital signs.
Other quality improvement initiatives, driven partly by the results of this review, have fully addressed a further 5/31 (16%) of the identified root causes and have realized some improvement in 3/31 (10%) others. One (3%) of the 31 identified root causes is subject to a process improvement, which is currently in progress, and 10/31 (32%) root causes have not yet been addressed. Communication about postoperative pain management has been improved during inter-unit transfers and between surgeons and anesthesiologists. This is by means of a beginning of day communication regarding cases on the operating room schedule and a structured handover between the operating room and the PICU. The APS take responsibility for a wider range of surgical patients. Some key nursing guidelines have been introduced or improved with the aim of: managing intravenous ketamine infusions in the ward setting; performing intravenous status and site-to-source checks of infusion systems; ensuring adequate pain control and appropriate observation following opioid bolus administration (including monitoring vital signs every ten minutes for 30 min) and before transferring a patient to another unit; and limiting intravenous lines to one per limb to decrease the probability of extravasation and interstitial infiltration. A standard policy for identifying and recording ideal body weight is being introduced and will be used for prescribing opioids in overweight patients. A study has been started to evaluate an acoustic respiratory monitor to improve early warning of respiratory depression for patients receiving opioid infusions.
There are limitations associated with this study. Identifying potential incidents retrospectively was difficult. The PSLS and Pharmacy Department naloxone lists may not have yielded a comprehensive list of incidents. The APS database, which is not ideally set up to trigger identification of incidents, was used only as a source of further information about selected cases. One hundred and eight of the 166 charts identified in our initial case selection contained no evidence of an actual incident (possibly because the naloxone withdrawal was recorded in error or because the notes in the PSLS entry were unclear), which highlights the lack of sensitivity in our retrospective identification of potential incidents.
There were some necessary limits set on the scope of the study because of time and resource constraints. The study, initiated by the BCCH APS, focused on infusions as the mode of drug administration for which the APS are primarily responsible. Cases in which opioids were administered by bolus dose only were excluded; these would also have been considerably more challenging to identify. Cases with a SAC score < 8 were not selected for RCA because of a low severity or low probability of recurrence; nonetheless, these cases may have contained important lessons.
There are inherent limitations in conducting a retrospective RCA. For example, good quality data, sufficient for analysis of root causes, is not always available in patient records or in voluntary reporting systems, such as PSLS, and identifying and contacting staff involved is difficult and may be associated with memory lapses or biases. Furthermore, despite the best efforts of the investigators and the use of a variety of data sources, it is difficult to ensure that all relevant incidents have been included within the study. Not all critical incidents are reported by healthcare staff. A recent discharge survey suggests that only 2.5% of adverse events reported by families are also reported by healthcare providers.28
Nonetheless, the RCA method provided a useful framework for this retrospective analysis. This review produced a range of recommendations to improve the safety of acute pain management in children. These were specific to BCCH but may have broader applicability. We are planning to complete a follow-up review using the CPSI’s revised incident analysis framework when the new policy of standard opioid concentrations has been in place for two years (mid-2014). Furthermore, prospective RCA data will be collected on future incidents as part of a continuous quality improvement program. This has been done for two incidents that have occurred since this audit was begun.
In this review, 5/14 (36%) of the patients in the RCA cases were infants (less than one year old). Only one of these five infants was managed by the APS from the outset. The consequences of opioid-related incidents may be particularly severe in neonates and infants who are at greater risk of opioid-induced respiratory depression and medication dose errors.7 All patients on opioid infusions in this vulnerable group should be attended by physicians with specialist knowledge of the developmental and pharmacogenetic variability of opioid medications in pediatric practice.
Inadequate analgesia was a contributing factor in 4/14 (29%) incidents for which a RCA was conducted. The short- and long-term consequences of poorly managed acute pain are well established,29-33 yet none of the identified patients had received continuous APS care throughout their hospital stay. It has been reported that patients receive as little as 55% of the services from which they would have benefitted,34 and yet, “errors of omission” may have more serious health consequences than “errors of commission”.35
There was a relatively high number of incidents occurring with COIs compared with PCA or epidural infusions, but the significance of this is hard to assess in the absence of accurate denominator data for COIs and is further complicated by the policy of COIs being managed by non-APS services while PCA and epidurals fall under the care of APS. Similarly, incomplete denominator data complicated the planned analysis of the impact of the APS on opioid critical incidents. Some errors may have been avoided if pain management had been conducted from the outset by a team with the resources to provide high-quality continuous care; 13/14 (93%) analyzed incidents were attributed in part to “Training” or “Communication” issues. Some of the APS’ specialist knowledge in pediatric acute pain management could be disseminated more widely. Root cause analysis suggested improvements to the education of residents and other healthcare professionals, particularly with respect to the pharmacogenetic variability of opioid medications in children. Notwithstanding detailed handover and documentation procedures, sharing patient care between different teams or transferring a patient between different units creates scope for loss of information and an increased risk of errors. Mitigating this risk should be a priority. Studies suggest that management of acute pain by a dedicated pain team can reduce the number of complications36 and increase patient safety and comfort,37,38 and that it is essential for the management of acute pain in children.13,39
In conclusion, this retrospective five-year review has illuminated 31 root causes contributing to the occurrence of opioid-related critical incidents within a tertiary care pediatric hospital. The results of this audit have provided a developed understanding of the relevant organizational practices and have contributed recommendations for improvement, several of which have been realized in recent institutional policy initiatives. A follow-up review will reveal how far these revised procedures have contributed to increased patient safety.
References
Canadian Association of Paediatric Health Centres & Institute for Safe Medication Practices Canada. Canadian Paediatric High Alert Medication Delivery: Opioid Safety; Toward a Change in Practice; Phase 2 Report January 28, 2010. Available from URL: www.caphc.org/s/2010-04-09-Final-Opiod-Report.pdf (accessed April 2013).
Canadian Patient Safety Institute; Paul J, Buckley N, McLean R, Musson D, Paul L, Antoni K (Investigators). Using Root Cause Analysis to Reduce Adverse Events on an Acute Pain Service. January 4, 2010. Available from URL: http://www.patientsafetyinstitute.ca/English/research/cpsiResearchCompetitions/2006/Documents/Paul/Paul Full Report.pdf (accessed April 2013).
Tesler MD, Wilkie DJ, Holzemer WL, Savedra MC. Postoperative analgesics for children and adolescents: prescription and administration. J Pain Symptom Manage 1994; 9: 85-95.
Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Critical Care Med 1999; 27: 2786-91.
Bouwmeester NJ, van den Anker JN, Hop WC, Anand KJ, Tibboel D. Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth 2003; 90: 642-52.
Institute for Safe Medication Practices Canada. National Collaborative: Top 5 Drugs Reported as Causing Harm through Medication Error in Paediatrics. ISMP Canada Safety Bulletin 9(6), 2009: 1-2. Available from URL: http://www.ismp-canada.org/download/safetyBulletins/ISMPCSB2009-6-NationalCollaborative-Top5DrugsReported.pdf (accessed April 2013).
Morton NS, Errera A. APA national audit of pediatric opioid infusions. Paediatr Anaesth 2010; 20: 119-25.
McDonnell C. Opioid medication errors in pediatric practice: four years’ experience of voluntary safety reporting. Pain Res Manag 2011; 16: 93-8.
Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Opioid-induced respiratory depression in paediatrics: a review of case reports. Br J Anaesth 2013; 110: 175-82.
Barletta JF. Clinical and economic burden of opioid use for postsurgical pain: focus on ventilatory impairment and ileus. Pharmacotherapy 2012; 32(9 Suppl): 12S-8S.
McDonald AJ, Cooper MG. Patient-controlled analgesia: an appropriate method of pain control in children. Paediatr Drugs 2001; 3: 273-84.
Esmail Z, Montgomery C, Courtrn C, Hamilton D, Kestle J. Efficacy and complications of morphine infusions in postoperative paediatric patients. Paediatr Anaesth 1999; 9: 321-7.
Verghese ST, Hannallah RS. Acute pain management in children. J Pain Res 2010; 3: 105-23.
Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med 1992; 326: 1-9.
Cass LJ, Howard RF. Respiratory complications due to inadequate analgesia following thoracotomy in a neonate. Anaesthesia 1994; 49: 879-80.
Woods D, Holl JL, Shonkoff JP, Mehra M, Ogagta ES, Weiss KB. Child-specific risk factors and patient safety. J Patient Saf 2005; 1: 17-22.
Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 2—Clinical use. Paediatr Anaesth 1997; 7: 93-101.
Anderson BJ, Persson MA, Anderson M. Rationalising intravenous morphine prescriptions in children. Acute Pain 1999; 2: 59-67.
Knibbe CA, Krekels EH, van den Anker JN, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet 2009; 48: 371-85.
European Medicines Agency. Evaluation of Medicines for Human Use - Assessment of the Paediatric Needs - Pain. June 2005. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/10/WC500004037.pdf (accessed April 2013).
Percarpio KB, Watts BV, Weeks WB. The effectiveness of root cause analysis: what does the literature tell us? Jt Comm J Qual Patient Saf 2008; 34: 391-8.
Canadian Patient Safety Institute; Hoffman C, Beard P, Greenall J, U D, White J. Canadian Root Cause Analysis Framework: A Tool for Identifying and Addressing the Root Causes of Critical Incidents in Healthcare - 2006. Available from URL: http://www.paediatricchairs.ca/safety_curriculum/domain6.docs/CPSIRootCauseAnalysisFramework.pdf (accessed April 2013).
Incident Analysis Collaborating Parties. Canadian Incident Analysis Framework. Edmonton, AB; 2012. Available from URL: www.ismp-canada.org/rca (accessed April 2013).
DeRosier J, Stalhandske E, Bagian JP, Nudell T. Using health care Failure Mode and Effect Analysis: the VA National Center for Patient Safety’s prospective risk analysis system. Jt Comm J Qual Improv 2002; 28: 248-67, 209.
US Department of Veterans Affairs. Safety Assessment Code (SAC) Matrix - 2012. Available from URL: http://www.patientsafety.gov/SafetyTopics/HFMEA/HFMEA_SAC.html (accessed April 2013).
Matlow AG, Baker GR, Flintoft V, et al. Adverse events among children in Canadian hospitals: the Canadian Paediatric Adverse Events Study. CMAJ 2012; 184: E709-18.
Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Ann Emerg Med 2007; 49: 1-8.
Daniels JP, Hunc K, Cochrane DD, et al. Identification by families of pediatric adverse events and near misses overlooked by health care providers. CMAJ 2012; 184: 29-34.
Sutters KA, Miaskowski C. Inadequate pain management and associated morbidity in children at home after tonsillectomy. J Pediatric Nurs 1997; 12: 178-85.
McCaffrey M, Pasero C. Pain: Clinical Manual, 2nd Edition. St. Louis: Mosby; 1999.
Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain 2011; 152: 904-11.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618-25.
Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101: 77-86.
McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003; 348: 2635-45.
Institute of Medicine. Patient Safety: Achieving a New Standard for Care. Aspden P, Corrigan J, Wolcott J, Erickson SM (Eds). Washington, DC: The National Academies Press; 2004.
Anderson BJ, McKenzie DR, Persson MA, Garden AL. Safety of postoperative paediatric analgesia. Acute Pain 1998; 1: 14-20.
Wheatley RG, Madej TH, Jackson IJ, Hunter D. The first year’s experience of an acute pain service. Br J Anaesth 1991; 67: 353-9.
Gould TH, Crosby DL, Harmer M, et al. Policy for controlling pain after surgery: effect of sequential changes in management. BMJ 1992; 305: 1187-93.
Lonnqvist PA, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth 2005; 95: 59-68.
Acknowledgements
The authors sincerely thank Warren Hill (Patient Safety, Quality & Accreditation leader during the study period), Ciara McGeough (Clinical Nurse Educator), Kevin Mui (Drug Utilization Pharmacist), and both Lolita Sarmiento and Anna Hong (research facilitators in Records Management) for their advice and assistance without which this review would not have been possible.
Conflicts of interest
None declared.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Nicholas West and Vahid Nilforushan participated in the literature review, root cause analysis, and drafting the submitted manuscript. Nicholas West, Vahid Nilforushan, and Jonathan Stinson participated in the data collection. Nicholas West provided the quantitative analysis. Vahid Nilforushan developed the methods of analysis. Jonathan Stinson participated in the data management, and the data analysis. J. Mark Ansermino advised on the study design, data analysis, and the presentation of the data. Gillian Lauder, the primary investigator, developed the initial study concept and design and guided the data collection and root cause analysis.
Rights and permissions
About this article
Cite this article
West, N., Nilforushan, V., Stinson, J. et al. Critical incidents related to opioid infusions in children: a five-year review and analysis. Can J Anesth/J Can Anesth 61, 312–321 (2014). https://doi.org/10.1007/s12630-013-0097-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0097-2