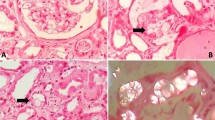
Abstract
Increased incidence of chronic kidney disease (CKD) with consecutive progression to end-stage renal disease represents a significant burden to healthcare systems. Renal tubulointerstitial fibrosis (TIF) is a classical hallmark of CKD and is well correlated with the loss of renal function. The bioactive lysophospholipid lysophosphatidic acid (LPA), acting through specific G-protein-coupled receptors, was previously shown to be involved in TIF development in a mouse model of unilateral ureteral obstruction. Here, we study the role of LPA in a mouse subjected to subtotal nephrectomy (SNx), a more chronic and progressive model of CKD. Five months after surgical nephron reduction, SNx mice showed massive albuminuria, extensive TIF, and glomerular hypertrophy when compared to sham-operated animals. Urinary and plasma levels of LPA were analyzed using liquid chromatography tandem mass spectrometry. LPA was significantly increased in SNx urine, not in plasma, and was significantly correlated with albuminuria and TIF. Moreover, SNx mice showed significant downregulation in the renal expression of lipid phosphate phosphohydrolases (LPP1, 2, and 3) that might be involved in reduced LPA bioavailability through dephosphorylation. We concluded that SNx increases urinary LPA through a mechanism that could involve co-excretion of plasma LPA with albumin associated with a reduction of its catabolism in the kidney. Because of the previously demonstrated profibrotic activity of LPA, the association of urinary LPA with TIF suggests the potential involvement of LPA in the development of advanced CKD in the SNx mouse model. Targeting LPA metabolism might represent an interesting approach in CKD treatment.




Similar content being viewed by others
References
Aikawa S, Hashimoto T, Kano K, Aoki J (2015) Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem 157:81–89
Alderton F, Darroch P, Sambi B, McKie A, Ahmed IS, Pyne N, Pyne S (2001) G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J Biol Chem 276:13452–13460
Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781:513–518
Benesch MG, Tang X, Venkatraman G, Bekele RT, Brindley DN (2015) Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J Biomed Res 30. doi:10.7555/JBR.29.20150058
Brindley DN, Pilquil C (2009) Lipid phosphate phosphatases and signaling. J Lipid Res 50(Suppl):S225–S230
Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM (2011) Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum 63:1405–1415
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, Levin A (2013) Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382:158–169
Eddy AA, Lopez-Guisa JM, Okamura DM, Yamaguchi I (2012) Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol 27:1233–1247
Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, Hudson BG, Caprioli RM (2014) Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res 55:1375–1385
Hara S, Kobayashi N, Sakamoto K, Ueno T, Manabe S, Takashima Y, Hamada J, Pastan I, Fukamizu A, Matsusaka T, Nagata M (2015) Podocyte injury-driven lipid peroxidation accelerates the infiltration of glomerular foam cells in focal segmental glomerulosclerosis. Am J Pathol 185:2118–2131
He J, Xu Y, Koya D, Kanasaki K (2013) Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol 17:488–497
Iwano M, Neilson EG (2004) Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens 13:279–284
Laouari D, Burtin M, Phelep A, Martino C, Pillebout E, Montagutelli X, Friedlander G, Terzi F (2011) TGF-alpha mediates genetic susceptibility to chronic kidney disease. J Am Soc Nephrol 22:327–335
Louis K, Hertig A (2015) How tubular epithelial cells dictate the rate of renal fibrogenesis? World J Nephrol 4:367–373
Pillebout E, Burtin M, Yuan HT, Briand P, Woolf AS, Friedlander G, Terzi F (2001) Proliferation and remodeling of the peritubular microcirculation after nephron reduction: association with the progression of renal lesions. Am J Pathol 159:547–560
Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, Schanstra JP (2007) LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 18:3110–3118
Rancoule C, Pradere JP, Gonzalez J, Klein J, Valet P, Bascands JL, Schanstra JP, Saulnier-Blache JS (2011) Lysophosphatidic acid-1-receptor targeting agents for fibrosis. Expert Opin Investig Drugs 20:657–667
Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14:45–54
Treguer K, Dusaulcy R, Gres S, Wanecq E, Valet P, Saulnier-Blache JS (2013) Influence of secreted factors from human adipose tissue on glucose utilization and proinflammatory reaction. J Physiol Biochem 69:625–632
Tsutsumi T, Adachi M, Nikawadori M, Morishige J, Tokumura A (2011) Presence of bioactive lysophosphatidic acid in renal effluent of rats with unilateral ureteral obstruction. Life Sci 89:195–203
Verdoorn KS, Lindoso RS, Lowe J, Lara LS, Vieyra A, Einicker-Lamas M (2010) Bone marrow mononuclear cells shift bioactive lipid pattern in injured kidney towards tissue repair in rats with unilateral ureteral obstruction. Nephrol Dial Transplant 25:3867–3874
Yang HC, Zuo Y, Fogo AB (2010) Models of chronic kidney disease. Drug Discov Today Dis Models 7:13–19
Acknowledgments
This work was supported by grants from INSERM and the Société Francophone du Diabète (grant 2015). We thank Christine Delage and Denis Calise (INSERM US06 CREFRE, Toulouse, France) for SNx surgery. Koryun Mirzoyan is supported by a Ph.D. grant from Europe (ERASMUS MUNDUS, MEDEA). All authors participated in the conception and design, or analysis and interpretation of the data, contributed to drafting and revising the manuscript, and gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted in accordance with National Institute of Health guidelines for the care and use of laboratory animals and were approved by a local ethics committee (CEEA#122).
Electronic supplementary material
ESM 1
(DOCX 194 kb)
Rights and permissions
About this article
Cite this article
Mirzoyan, K., Baïotto, A., Dupuy, A. et al. Increased urinary lysophosphatidic acid in mouse with subtotal nephrectomy: potential involvement in chronic kidney disease. J Physiol Biochem 72, 803–812 (2016). https://doi.org/10.1007/s13105-016-0518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0518-0