Abstract
Background
The viral persistence in patients with Coronavirus Disease 2019 (COVID-19) remains to be investigated.
Methods
We investigated the viral loads, therapies, clinical features, and immune responses in a 70-year patient tested positive for SARS-CoV-2 for 3 months.
Findings
The patient exhibited the highest prevalence of abnormal indices of clinical features and immune responses at the first admission, including fever (38.3 ℃), decreased lymphocytes (0.83 × 109/L) and serum potassium (3.1 mmol/L), as well as elevated serum creatinine (115 µmol/L), urea (8.6 mmol/L), and C-reactive protein (80 mg/L). By contrast, at the second and the third admission, these indices were all normal. Through three admissions, IL-2 increased from 0.14 pg/mL, 0.69 pg/mL, to 0.91 pg/mL, while IL-6 decreased from 11.78 pg/mL, 1.52 pg/mL, to 0.69 pg/mL, so did IL-10 from 5.13 pg/mL, 1.85 pg/mL, to 1.75 pg/mL. The steady declining trend was also found in TNF-α (1.49, 1.15, and 0.85 pg/mL) and IFN-γ (0.64, 0.42, and 0.27 pg/mL). The threshold cycle values of RT-PCR were 26.1, 30.5, and 23.5 for ORFlab gene, and 26.2, 30.6, and 22.7 for N gene, showing the patient had higher viral loads at the first and the third admission than during the middle term of the disease. The patient also showed substantially improved acute exudative lesions on the chest CT scanning images.
Conclusions
The patient displayed declining immune responses in spite of the viral shedding for 3 months. We inferred the declining immune responses might result from the segregation of the virus from the immune system.
Similar content being viewed by others
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a series of Coronavirus Disease 2019 (COVID-19) that was declared a pandemic by the World Health Organization (WHO) in March 2020. As of May 12, 2020, there have been over 4 million confirmed cases of COVID-19 and 280 thousand deaths globally [1]. The great part of asymptomatic COVID-19 cases and the high infectiousness of SARS-CoV-2 dramatically hinder the containing of COVID-19 [2]. Both the asymptomatic and symptomatic patients can shed a similar amount of virus [3], indicating both types of patients are equally effective in spreading COVID-19. On average, patients will cease to be tested positive for SARS-CoV-2 after 21 days from COVID-19 onset [4]. Among the clinically cured patients, a few became recurrent cases after discharge [5, 6]. To our knowledge, the longest time of positive PCR results for SARS-CoV-2 is 49 days from COVID-19 onset [7].
The patients with long-term positive PCR have a longer duration of virus-shedding than the asymptomatic patients, suggesting the presence of the persistent virus in patients who may develop into a chronic condition. Nonetheless, the SARS-CoV-2 RNA shedding pattern has not been well characterized, despite the suggestions that patients with COVID-19 may shed the viral nucleic acid in a manner like patients with influenza and unlike patients with SARS [8, 9]. In any case, understanding the temporal characteristics of viral shedding is crucial in the prevention and control of COVID-19. A previous study identified that sex, delayed hospital admission after illness onset and invasive mechanical ventilation were associated with prolonged SARS-CoV-2 shedding. However, these risk factors are generally used to explain a prolonged length of several days and are not comprehensive enough to explain the long-term viral shedding. From the research on other viruses that cause chronic diseases in human being, the possible mechanisms of persistent viruses include: CD8 cytotoxic T-lymphocytes (CTLs) lose their functionality due to inactivation and/or physical deletion of lymphocytes [10]; There exists a disruption of CD4 T-cell responses that fail to sustain CTL activity and to form CD8 T-cell memory during the persistent viral infection [11, 12].
As to the emerging COVID-19, it remains to be investigated whether there are mechanisms for patients to develop into a chronic condition, since viral persistence may considerably dampen the effectiveness of measures to control the transmission of COVID-19. Here, we report a special case with SARS-CoV-2 shedding for more than 3 months. We investigated the viral loads, therapies, clinical features, and immune responses of this patient during the different phases of disease progression. Our objectives were to understand the interactions between SARS-CoV-2 and the host immune responses, which might shed light on the cause of viral persistence in patients with COVID-19.
Methods and material
Study patient
This study was approved by the Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences (Certificate no. PJ-NBEY-KY-2020-061-01). Written consent was obtained from the patient. The 70-year-old male patient was admitted to the hospital on February 2 because of fever (38.3 ℃), intermittent cough, and rough breath in both lungs. He reported the first symptom of fatigue 3 days before admission. The patient had a weight of 70 kg, a height of 1.7 m, a history of hypertension for more than 20 years, and emphysema at admission. His blood pressure was well controlled at admission. The patient had no medical history of compromised immunity. He was diagnosed to have COVID-19 through the positive reverse transcription-PCR (RT-PCR) result for SARS-CoV-2 according to the diagnostic criteria released by the National Health Commission of China [13]. The date of illness onset was defined as the day when the first symptom occurred, and the date of diagnosis was defined as the day when SARS-CoV-2 was first detected.
RT-PCR
We conducted RT-PCR on the nasopharyngeal, blood, and rectal swab samples for possible viral shedding routes according to the recommendations mentioned above. The samples were collected and tested upon admission and then every 1–3 day throughout the hospitalization period. We used the cycle threshold (Ct) values of RT = PCR to approximately represented the viral load (inversely related to Ct value) in the patient. Two target genes, open reading frame1ab (ORF1ab) and nucleocapsid (N), were amplified by two sets of primers recommended by the National CDC (China) (https://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html). In addition, influenza-A (H1N1) virus, influenza B virus, respiratory syncytial virus, parainfluenza virus, and adenovirus were also tested with RT-PCR.
Data collection
We collected the clinical data of the patient by an experienced physician. The patient’s data included the epidemiological data that were obtained through standard epidemiological questionnaires and interviews, as well as the clinical data that consisted of symptoms, treatments, results of laboratory testing, computed tomography (CT) scans (Siemens Sensation 16-slice CT), and clinical outcomes. To assess the immune responses induced by SARS-CoV-2, we collected the blood sample on admission and measured white blood cells (WBC), lymphocytes, C-reactive protein (CRP), and cytokines that included IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ. Other laboratory tests consisted of coagulation profile, creatinine, blood urea nitrogen, alanine aminotransferase, aspartate transferase, creatine kinase, lactate dehydrogenase, electrolytes, and arterial blood gas analysis.
Results
Hospitalization and clinical features
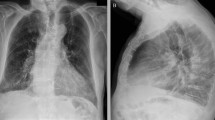
During the first hospitalization from February 2 to February 20, the patient showed the most severe condition among three hospitalizations, including fever (38.3 ℃), decreased lymphocytes (0.83 × 109/L) and serum potassium (3.1 mmol/L), as well as elevated serum creatinine (115 µmol/L) and urea (8.6 mmol/L) (Table 1). The patient was given antiviral therapy for COVID-19, including Umifenovir tablets, Lopinavir/Ritonavir tablets, and inhalation of recombinant human interferon-α 2b. No steroid therapy was administered. On February 20, the patient was discharged according to the criteria for the clinically cured patients: two consecutive nasopharyngeal swabs and one rectal swab tested negative for SARS-CoV-2 by RT-PCR, as well as normal temperature lasting longer than 3 days, resolved respiratory symptoms and substantially improved acute exudative lesions on chest CT scanning images (Fig. 1) [13]. After discharge, the patient was isolated at a designated site and was under close medical observation by a physician for 14 days.
On March 5, the patient was admitted for the second time when his nasopharyngeal swab was tested positive for SARS-CoV-2 during the routine follow-up. The clinical features mentioned above were all normal. The patient was treated with Abidor hydrochloride tablet, Darunavir and Cobistat tablets for antiviral treatment. He was discharged on March 16 according to the criteria mentioned already. During the follow-up after the second hospitalization, the patient was tested weakly positive both for SARS-CoV-2-IgM and SARS-CoV-2-IgG.
On April 25, the patient had a third admission when his nasopharyngeal swab was consecutively tested weakly positive for SARS-CoV-2 (on April 10, April 18, and April 25). He did not complain of any discomfort. On May 8, his nasopharyngeal swab was tested positive for SARS-CoV-2. He was given 50 mg Darunavir and Cobistat once a day orally. On May 17, he was discharged for the third time.
The patient’s CT scanning images showed a steady resolution of infection in the lung from the first to the third hospitalization (Fig. 1).
Viral load
The threshold cycle (Ct) values of RT-PCR were 26.1, 30.5, and 23.5 for ORFlab gene, and 26.2, 30.6, and 22.7 for N gene, respectively, which were inversely related to the viral load (Table 1). The RT-PCR results showed the patient had higher viral loads at the first and the third admission than during the middle term of the disease. The blood and fecal samples were all tested negative for SARS-CoV-2.
Immune response and inflammatory indices
The patient exhibited the highest prevalence of abnormal indices for immune response and inflammation at the first admission, including fever (38.3 ℃), decreased lymphocytes and elevated CRP (80 mg/L). By contrast, at the second and the third admission, these indices were all normal. Through three hospitalizations, IL-2 increased from 0.14 pg/mL, 0.69 pg/mL, to 0.91 pg/mL, while IL-6 decreased from 11.78 pg/mL, 1.52 pg/mL, to 0.69 pg/mL, so did IL-10 from 5.13 pg/mL, 1.85 pg/mL, to 1.75 pg/mL. The steady declining trend was also found in TNF-α (1.49, 1.15, and 0.85 pg/mL) and IFN-γ (0.64, 0.42, and 0.27 pg/mL). IL-4 did not display an apparent trend, as shown by three results of 1.24, 0.82 and 1.43 pg/mL.
Discussion
The study observed a declining immune reactivity in a patient with COVID-19 that persisted for 3 months despite the high viral load during the long duration of viral persistence.
Throughout the infection period, the viral load at the first and the third admission was all at high levels according to the Ct values. When the infection began, the immune reactivity in the patient was at the highest level, as judged from the significantly elevated CRP, and reduced lymphocytes, as well as the elevated IL-6 and IL-10 (Table 1). The high level of immune reactivity coordinated with the disease severity that was indicated by the increased serum creatinine and urea, which suggested renal dysfunction, as well as decreased potassium, which was reported to be present in severe cases [14]. The current results demonstrated that IL-2 was significantly affected and steadily recovered after the first infection (Table 1), meaning the activated human T cell populations were most reduced at the initial infection because IL-2 is a growth factor capable of driving the expansion of activated human T cell populations [15]. The changes in IL-2 concentrations were consistent with the changes in the numbers of lymphocytes during the three periods of infection. In contrast, the concentrations of IL-6 and IL-10 were highest when SARS-CoV-2 initially infected the patient. These cytokines are crucial in the immune responses to viral infection. IL-4 participated in B-cell activation maximally at the beginning of infection [16]; IL-6 is an endogenous pyrogen and mediator of the acute phase response [17]; and IL-10 is a key regulator of acute versus chronic infection [18]. The high levels of IL-6 and IL-10 at the first admission paralleled with the abnormalities in body temperature, lymphocyte counts, and CRP. IL-4 did not display an apparent trend. In addition, the study also demonstrated that both TNF-α and IFN-γ were secreted in the maximum amount at the first infection, suggesting the most powerful antiviral immunity occurred at the beginning of infection [19, 20]. Taken all the information together, although the viral load was at a higher level during the third hospitalization than during the first hospitalization, the immune responses were significantly weakened after a long period of infection. This reduced immunity might account for the persistent virus shedding.
SARS-CoV-2 can lead to a proinflammatory cytokine release via the angiotensin II pathway that is a possible therapeutic target via the IL-6-STAT3 axis [21]. The viral contact with the host systemic immune system is expected to provoke significantly elevated cytokines. As the patient had no medical history of compromised immunity and his apparent immune responses were observed during the first hospitalization, we hypothesized SARS-CoV-2 was separated from the patient’s systemic immune system, which could be partly supported by the absence of the virus in the blood and fecal samples throughout the infection. The steady resolution of infection in the lung indicated no further invasion by SARS-CoV-2 after the first hospitalization (Fig. 1). This type of COVID-19 progression, a long duration of viral shedding, and absent symptoms, indicated that the patient was not significantly affected by the persistent SARS-CoV-2 during the late phase of infection. The patient had emphysema, which might affect the structure of alveoli, which might provide a physical mechanism for the segregation of the virus from the immune system. To our knowledge, several older patients who were super spreader were reported in the news [22].
The limitation of the study was the difficulty to assess the treatment outcomes for a case report. The interferon was used along with Umifenovir and Lopinavir during the first hospitalization because the patient appeared to be in a severe condition then. During the second and the third hospitalization, only two or three antiviral agents were administered without interferon. Future studies about treatment choices are required for such special cases.
In conclusion, we observed a patient with COVID-19 who shed virus for 3 months and meanwhile displayed declining immune response. We concluded that the declining immune response might result from the segregation of the virus and the immune system. Further study is required to investigate patients with long-term shedding of SARS-CoV-2.
References
Wolrd Health Organization. (2020) Coronavirus disease (COVID-19) Situation Report-113. Retrieved at https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200512-covid-19-sitrep-113.pdf.
Qiu H, Wu J, Hong L, Luo Y, Song Q. Clinical epidemiological features of 36 children with coronavirus disease (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2019. https://doi.org/10.1016/S1473-3099(20)30198-5.
Zhou R, Li F, Chen F, Liu H, Zheng J, Lei C, et al. Viral dynamics in asymptomatic patients with COVID-19. Internat J Infect Dis IJID. 2020;96:288–90. https://doi.org/10.1016/j.ijid.2020.05.030.
Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England J Med. 2020;382:1177–9. https://doi.org/10.1056/NEJMc2001737.
Loconsole D, Passerini F, Palmieri VO, Centrone F, Sallustio A, Pugliese S, et al. Recurrence of COVID-19 after recovery: a case report from Italy. Infection. 2020. https://doi.org/10.1007/s15010-020-01444-1.
Zhang B, Liu S, Dong Y, Zhang L, Zhong Q, Zou Y, et al. Positive rectal swabs in young patients recovered from coronavirus disease COVID-19. J Infect. 2019. https://doi.org/10.1016/j.jinf.2020.04.023.
Tan L, Kang X, Zhang B, Zheng S, Liu B, Yu T, et al. A special case of COVID-19 with long duration of viral shedding for 49 days. Medrxiv. 2020;3:200. https://doi.org/10.1101/2020.03.22.20040071.
Tsang TK, Cowling BJ, Fang VJ, Chan KH, Ip DK, Leung GM, et al. Influenza A Virus Shedding and Infectivity in Households. J Infect Dis. 2015;212:1420–8. https://doi.org/10.1093/infdis/jiv225.
He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020. https://doi.org/10.1038/s41591-020-0869-5.
Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–14. https://doi.org/10.4049/jimmunol.172.7.4204.
Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–86. https://doi.org/10.4049/jimmunol.170.1.477.
Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–27. https://doi.org/10.1128/JVI.79.16.10514-10527.2005.
National Health Commission of the People’s Republic of China. (2019) Diagnosis and treatment guidelines for 2019 novel coronavirus pneumonia (Draft version 7)[EB/OL] (March 3, 2020). https://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml. (Accessed 5 Mar 2020)
Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3:e2011122.
Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18:648–59. https://doi.org/10.1038/s41577-018-0046-y.
Banchereau J. Generation of human B-cell lines dependent on CD40-ligation and interleukin-4. Front Immunol. 2015;6:55. https://doi.org/10.3389/fimmu.2015.00055.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Biol. 2014;6:a016295. https://doi.org/10.1101/cshperspect.a016295.
Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–6. https://doi.org/10.1016/j.tim.2007.02.006.
Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol Rev. 2013;255:125–48. https://doi.org/10.1111/imr.12086.
Sedger LM. microRNA control of interferons and interferon induced anti-viral activity. Mol Immunol. 2013;56:781–93. https://doi.org/10.1016/j.molimm.2013.07.009.
Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–3. https://doi.org/10.1016/j.immuni.2020.04.003.
Reinberg S (2020) ‘Super Spreader’ Events Increase COVID-19 Cases. https://www.ebmdcom/lung/news/20200409/super_spreader_events_increase_covid_19_cases#1.
Funding
This study was supported by the COVID-19 Platform Program of Hwa Mei Hospital, University of Chinese Academy of Sciences (2020HMZD21, 2020HMZD27).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethics statement
This study was approved by the Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences (Certificate no. PJ-NBEY-KY-2020-061-01) and followed the Declaration of Helsinki and its later amendments. Written consent was acquired from the patient.
Availability of data and material
With the permission of the corresponding authors, we can provide participant data without names and identifiers. Data can be provided after the Article is published.
Rights and permissions
About this article
Cite this article
Gao, G., Zhu, Z., Fan, L. et al. Absent immune response to SARS-CoV-2 in a 3-month recurrence of coronavirus disease 2019 (COVID-19) case. Infection 49, 57–61 (2021). https://doi.org/10.1007/s15010-020-01485-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-020-01485-6