Abstract
Cystinosis is a rare autosomal recessive disease with an incidence of approximately 1 case per 100,000–200,000 live births. Over the years, gaining in-depth knowledge of the disease has led to vast improvement in patient life expectancy. However, debilitating, extra-renal manifestations such as eye disease, in particular corneal crystal deposition and its associated photophobia, still occur frequently, regardless of patient age and notwithstanding the increased implementation of systemic therapy. Ophthalmological assessment has not yet been standardized. The aim of this article was to provide clear recommendations for ophthalmological assessment during follow-up of patients with cystinosis to improve quality and regularity of ophthalmological care and thereby minimize ophthalmological complications. A literature search was performed to assess previous and current recommendations on examinations to conduct during follow-up of patients with cystinosis. Multidisciplinary cystinosis clinics were set up in collaboration with the Department of Ophthalmology and the Department of Pediatric Nephrology to allow patients to be seen by a nephrologist, an ophthalmologist and other specialists on the same day. Based on the results of these multidisciplinary clinics the standardized clinical ophthalmological assessment was drafted. This is a protocol for follow-up, describing the approach taken regarding ophthalmological follow-up of patients with cystinosis, considering the different types of the disease and the time since diagnosis. Standard examination includes history, visual acuity, tonometry and slit-lamp examination, with fundus photography performed at diagnosis and annually thereafter. Confocal microscopy is the imaging modality of choice, while anterior segment optical coherence tomography (OCT) is a good alternative. Finally, posterior segment OCT for imaging of the macular region and optic nerve should be conducted on an annual basis.
Similar content being viewed by others
Introduction
Cystinosis is a rare autosomal recessive lysosomal storage disease, with an incidence of approximately 1 case per 100,000–200,000 live births [1]. The disorder results from mutations in the CTNS gene, mapped on chromosome 17p13.2, which encodes the protein cystinosin, the lysosomal membrane transporter responsible for cystine egress [2]. Impairment of cystinosin leads to the accumulation of cystine within lysosomes and the formation of characteristic crystals. The accumulation in numerous tissues, including the kidneys, eyes, bone marrow, liver, spleen, pancreas, thyroid, muscle and brain causes various clinical symptoms [2, 3].
Patient cases of cystinosis were first reported by Abderhalden in 1903 [4]. However, clinical understanding of the disease did not progress until the early 1930s, when Fanconi, deToni and Debré individually described a generalized renal tubular disorder in children, de Toni–Debré–Fanconi syndrome, or Fanconi syndrome [1]. The modernization of clinical research in the 1960s led to the identification of the amino acid cystine in tissue samples of affected children [5] and by 1982, further research determined that an impairment of lysosomal membrane transport was the basic defect in cystinosis [6]. To date, more than 100 CTNS mutations have been elucidated, the most common in the North European population being the 57-kB deletion [7].
Clinical Characteristics
Clinically, there are three different types of cystinosis, the most frequent (95% of cases) and severe type being the classic infantile cystinosis (Figs. 1, 2). It is the most common inherited cause of renal Fanconi syndrome in children and, if left untreated, it leads to failure to thrive, growth retardation and inevitably to end-stage renal failure, prior to the end of their first decade of life [8]. The intermediate nephropathic form is less frequent and presents with milder symptoms compared to the classic infantile form, at an older age during childhood, adolescence or even adulthood. These patients do have renal impairment but do not suffer from growth retardation or profound tubular dysfunction. Lastly, the non-nephropathic or ocular cystinosis, the least frequent form, is characterized only by photophobia, due to corneal crystals, without systemic involvement [8]. In 1976, Thoene and colleagues [9] described the cysteine-depleting properties of oral cysteamine. Treatment with cysteamine resulted in the formation of a cysteamine-cysteine mixed disulfide compound, which is able to exit the lysosome via the PQ-loop repeat-containing protein 2 (PQLC2) transporter [10]. The introduction of cysteamine as systemic treatment and pediatric renal replacement therapy provided the possibility for these patients to survive.
Early treatment with oral cysteamine proved to reduce and delay deterioration of renal function, improve growth and prevent late non-renal complications [3, 11].
With the advent of both therapies, there was a dramatic change in the clinical course of cystinosis. Life expectancy of patients significantly improved. This general improvement of patient’s health has transformed our approach to complex complications in non-renal tissues [12]. Currently, corneal crystal deposition resulting in photophobia and retinopathy potentially leading to blindness are two of the most troublesome ocular complications affecting patients with cystinosis. The cystinosis patient nowadays should receive early ophthalmological care in order to reduce not only corneal involvement, but also to prevent detrimental retino- and opticopathy, which can have great impact on patient quality of life.
Ocular Manifestations
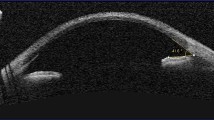
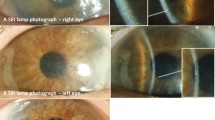
The eye can be considered as the window to cystinosis, reflecting its severity through its glassy pane, the cornea [2]. In 1941, Burki was first to describe the presence of cystine crystals within the cornea and conjunctiva as a myriad of small, white, shining sequin-like crystals, which are deposited on the superficial layers of the parenchyma, while respecting the limits of the epithelium and endothelium [13]. These needle-shaped, highly reflective cystine crystals are pathognomonic for cystinosis and can be visualized by slit-lamp examination [14]. According to Gahl and colleagues, in patients with the classic infantile form, the crystals are visible from the age of 16 months onwards but can be visible earlier by an experienced ophthalmologist. They accumulate progressively in the first few years of life, but at different rates in different patients. Corneal crystal deposition begins in the anterior peripheral cornea and progresses more centrally and deeper into the stroma [15]. For many years, it was not known if the endothelium was also involved, but better visualizing techniques, such as confocal microscopy, confirmed packing of crystals in the epithelium and the stroma, but not in the endothelium [16]. The accumulation of corneal crystals progresses with age, rarely affects central vision, but is considered responsible for many debilitating symptoms. The first and most frequently reported ocular symptom in cystinosis is photophobia [17]. Photophobia may be due to the accumulation of corneal cystine crystals but its exact pathophysiology in relation to crystal deposition and corneal tissue change remains unclear [2]. Recurrent corneal erosions, blepharospasm, chronic red eye, superficial punctate or filamentary keratopathy with ensuing foreign body sensation and band keratopathy or glaucoma can also complicate long-standing cystinosis [12]. The differential diagnosis of corneal cystinosis crystals [18] includes diseases with crystalline-like deposits such as tyrosinemia, gout, multiple myeloma [19] and Bietti crystalline corneal dystrophy. Acquired depositions from drugs such as gold in chrysiasis, silver in argyriasis, can also result in corneal deposits but are less frequent nowadays. Hypolipoproteinemias such as fish eye disease, lecithin-cholesterol acyltransferase-deficiency (LCAT) and Tangier disease should be considered, especially in young children. In the 1960s and 1970s, cystinotic retinopathy, described as patches of depigmentation with pigmentary mottling progressing posteriorly, and a maculopathy with decreasing visual acuity were regularly described. Crystals have been reported to appear in the anterior chamber, in the uvea and optic nerve [20, 21]. Awareness of symptoms caused by primary intracranial hypertension such as episodes of acute visual loss or optic nerve involvement is crucial. This possible association was described in cystinosis patients with an Arnold–Chiari anomaly, chronic kidney disease, initiation of growth hormone therapy, tapering of corticosteroids and possibly suboptimal disease control [22].
The mainstay of cystinosis therapy, oral administration of cysteamine, reduces systemic cystine accumulation. In developed countries, all patients are now systematically treated with oral medication. This combined with the fact that the retina is a highly vascularized structure means that the rate of maculopathy and retinopathy has substantially declined. However, oral cysteamine has no effect on corneal cystine crystals [23], because of its avascular nature. Topical treatment with standard cysteamine hydrochloride (CH) eye drops (0.1%) has long been shown to be effective in reducing corneal crystal density and alleviating symptoms [24].
However, several problems are associated with this formulation, including frequency of administration, stability and bioavailability. It is recommended to administer the cysteamine hydrochloride eye drops hourly during waking hours, starting after diagnosis [14, 25, 26]. This is difficult to achieve for most patients, and those who are unable to adhere to this regimen are encouraged to use the drops at least six times a day. Furthermore, cysteamine oxidizes to disulfide at room temperature [27], loosing potency [28] and releasing an unpleasant odor in the process. In addition, some patients complain that it causes a stinging sensation when applied. A more viscous formulation (CH 0.55%) has recently been developed (Cystadrops®, Orphan Europe, Recordati, Puteaux, France; granted marketing approval within the European Union in October 2016) [29]. It is as effective and safe in reducing corneal cystine crystal density and superior to treatment with cysteamine hydrochloride 0.10% drops and only requires administration four times a day [30].
In rare diseases, such as cystinosis, there is a lack of standardized guidelines for ophthalmological assessment and appropriate follow-up of patients. The aim of this article was to produce such a guideline. The current literature was reviewed and findings were combined with clinical experience to produce a document to guide scheduling of follow-up visits and present a protocol for ophthalmological examination for patients with cystinosis.
Methods
Literature Search
A Medline search was carried out by two authors (AMP and MTH) who independently performed different and parallel searches in September 2015. The main keywords “cystinosis”, “follow-up”, “corneal crystal load”, “ophthalmological complications”, and “treatment” were used to outline the scope of the literature. The search terms were used for all fields (title, abstract, keywords and full text) and all article types were included. To supplement the search, expert opinion was sought during the Ophthalmological Symposium on Cystinosis in Salzburg in October 2015. Further sources were identified by searching reference lists to identify relevant studies and by following internal citations. The search was repeated for new references in March 2016, September 2016 and April 2017.
Clinical Setting
Multidisciplinary cystinosis clinics were established by combining appointments for the nephrology and ophthalmology departments of the University Hospitals Leuven, which allowed patients to be seen by a nephrologist, ophthalmologist and other specialists on the same day. All patients with cystinosis treated at the University Hospitals Leuven were routinely included in these clinics. At the end of the first and second follow-up appointments, all patients and their companions were asked to give feedback (positive and negative) on the process, which was then recorded in their file.
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Literature Search Results
Nearly 70 articles in English were identified and their content was examined. We selected peer-reviewed articles, preferably published in a high-impact factor journal. An exception was made for some articles in specialized journals because some of these have lower-impact factors. This literature review served as a basis for generating a follow-up ophthalmological assessment for patients with cystinosis.
Creation of a Follow-Up Assessment Schedule
A follow-up assessment schedule, for use within the multidisciplinary clinics, was created in order to structure follow-up consultations for patients with different types of cystinosis (Fig. 3). Given most patients are not familiar with the disease at diagnosis, we propose seeing them four times in the first year after diagnosis. This was to guide ocular treatment, listen to the patients’ experience and to be able to inform them on a regular basis about the special ophthalmological needs, promote patient self-care and motivate them to adhere to the ocular cysteamine treatment. These consultations at diagnosis, 3-, 6- and 12-months post diagnosis were simultaneously held at the nephrology department to minimize hospital visits and maximize patient support. Slightly different follow-up schedules were derived depending on the time after diagnosis to familiarize patients with eye examination and not burden them with difficult examinations too soon (Fig. 3).
The standardized clinical ophthalmological assessment, a protocol for follow-up of patients with cystinosis. AS-OCT anterior segment optical coherence tomography, CM confocal microscopy, FE fundus examination, FP fundus photography, SE standard examination, PS-OCT posterior segment optical coherence tomography, SP split-lamp photography. Standard examination (SE) includes history, visual acuity, tonometry and slit-lamp examination
Ophthalmological Assessment
After reviewing the literature, an extensive number of publications on the many different diagnostic tests for cystinosis were found. These included visual acuity measurements, slit-lamp photography, IOP measurement, corneal pachymetry, corneal sensation, specular microscopy, gonioscopy, ultrasound biomicroscopy, dilated fundus exams, as well as technical examinations (ERG, ICVM, OCT, perimetry, corneal pachymetry). However, there were no detailed, up-to-date articles on regular ophthalmological follow-up of patients with cystinosis. Below, we propose our methods of assessment, which provide a more in-depth ophthalmological analysis of the cystinosis patient.
Medical History
Obtaining a full medical history from every cystinosis patient is crucial. Objective data, such as clinical type of cystinosis and the responsible mutation, the presence of positive familial history, familial or personal history of glaucoma and medications, especially systemic cysteamine medication, should be carefully recorded. Secondly, a history of the patient’s personal experience concerning sharpness of vision, grade of photophobia, quality of night vision and eventual pain should be obtained, as indicated in Table 1.
Given its subjective nature, the degree of photophobia is a difficult parameter to measure accurately. Therefore, a self- and clinician-assessed evaluation of photophobia scaling system is used (Table 2; previously published for vernal keratoconjunctivitis or other inflammatory ocular surface diseases) [17, 31]. Liang and colleagues recently published this scoring scale in cystinosis patients [17]. They evaluated clinician-assessed photophobia with slit-lamp biomicroscopy with a score modified from a previous study [17].
Visual Acuity
Best-corrected visual acuity (BCVA) at distance is measured using a Snellen chart at 6 m. BCVA for near visions is tested one eye at a time using the Parinaud scale.
Tonometry
Intraocular pressure is measured using a portable rebound tonometer (Icare®, Icare Finland Oy, Helsinki, Finland) [32] or the standard Goldmann tonometer.
Slit Lamp Biomicroscopy
A summary of the slit-lamp assessment is presented in Table 3.
Examinations of the eyelids and conjunctiva are performed first, followed by inspection of the cornea, iris and lens. Regarding the conjunctiva, the presence of cystine crystals is noted and the level of bulbar conjunctival hyperaemia is quantified using the Cornea and Contact Lens Research Unit (CCLRU) grading scale. The observer quantifies the degree of bulbar conjunctival redness at the slit lamp in comparison to the CCLRU hyperemia standards, which provide a set of photographic images with four degrees of redness; very slight, slight, moderate, severe. Corneal fluorescein staining is scored using the Oxford scale in which staining is represented by punctate dots on a series of panels (A–E). Staining ranges from 0–5 for each panel [33]. Evidence for filamentous or band keratopathy is also recorded. In case of peripheral corneal neovascularization, clock hours are noted. Complications associated with the iris, such as iris transillumination, crystal deposition and posterior synechiae are also investigated. Biomicroscopy is documented by slit-lamp photographs.
The slit-lamp scoring system or corneal cystine crystal score (CCCS), created in 2000 by Gahl and colleagues and based on slit-lamp photographs of corneas with increasing cystine crystal densities (0.00–3.00), is the oldest scoring system and has long been widely used to assess the degree of crystal accumulation in patients with nephropathic cystinosis [2].
Fundus Examination
After dilation with tropicamide 0.5% and phenylephrine 10%, the fundus is inspected for crystals on the retinal surface, depigmentation of the retina and alterations of the pigment epithelium. Optic disc, macula and vessels are scored. In case of abnormal appearance, details are noted. Documentation of the fundus is performed with an ultra-wide field fundus camera (Optos®, Marlborough, MA; Table 4).
Technical Examinations
The best imaging technique to identify and to quantify cystine crystals in the cornea is in vivo confocal microscopy (IVCM). IVCM is an optical imaging technique that can quantify crystal density in all corneal layers at the cellular level. A posterior segment optical coherence tomography (PS-OCT) is also performed for each patient to examine the macula.
Flash electroretinogram (ERG) was once a method to examine retinal function in cystinosis patients. Since all patients in the clinic are on oral medication, retinal problems are rare; therefore, we do not recommend regular follow-up with ERG, except if symptoms emerge.
Follow-up Assessment Schedule
Both infantile and juvenile cystinosis are chronic diseases requiring regular hospital visits. The multidisciplinary clinics of the nephrology and ophthalmology departments allow patients with cystinosis to be seen by different specialists on the same day.
Based on clinical expertise regarding patient care and motivation and medical follow-up of patients with corneal crystal accumulation, an examination schedule was created—the standardized clinical ophthalmological assessment (Fig. 3). This schedule takes into account the different types cystinosis and the time since diagnosis. It was composed as a supporting structure in order to achieve regular and consistent follow-ups and provide a guide to all ophthalmology and nephrology specialists in our hospital. Furthermore, it also serves a guide for the patient by providing information on what to expect when going for a routine follow-up visit. This schedule was produced to prevent challenges in cystinosis follow-up and achieve better results, both physically and mentally with a focus on the patient.
In patients with infantile or juvenile cystinosis, a first ophthalmological exam is conducted at diagnosis, 3 months post-diagnosis, and every 6 months from then on; ocular cystinosis only requires annual follow-up. Standard examination (SE) includes history, visual acuity, tonometry and slit-lamp examination. Depth of corneal crystal deposition is quantified subjectively at 0–25–50–75–100% of central and peripheral corneal thickness deposition. Fundus photography should be performed at diagnosis and annually thereafter. The technical examination should be adapted to the devices the ophthalmologist has access to. However, confocal microscopy is the imaging modality of choice, if available, although this examination is not to be performed at the first visit. If confocal microscopy is not available, anterior segment optical coherence tomography (AS-OCT) is a good alternative. Finally, it is recommended to conduct PS-OCT for imaging of the macular region, on an annual basis.
Semi-annual follow-up appointments are scheduled at the University Hospitals Leuven after the first year post-diagnosis. A complete ophthalmological examination, which contains an SE, conducting OCT while dilating, followed by a fundus examination takes approximately 1 h and 20 min per section.
Multidisciplinary Clinics and Patient Feedback
Of the patients old enough to understand the concept of the multidisciplinary clinics, 100% reacted positively. In younger patients, mainly the parents or other people who accompanied them, were satisfied with the arrangement. They reported, in particular, the advantage of knowing in advance which examinations awaited them and how much time they would take. Patients and their families also highly rated the fact that both the ophthalmology and nephrology appointments were scheduled for the same day, which halved the amount of hospital visits per year. Because the response from patients was unanimously positive, no alterations to the schedule were made based on this feedback.
Discussion
The literature research did not yield a comprehensive article that focused solely on the ophthalmological follow-up and summarized the cost- and time-effective methods for follow-up. Furthermore, many of the diagnostic tests described in the literature, such as ERG, perimetry and pachymetry, have been outmoded by confocal microscopy and OCT. A 2015 article by a Spanish group in Nefrologia [34] provides excellent general guidelines for the treatment of patients with cystinosis. This article is, however, more focused on the nephrological rather than ophthalmological element and does not provide in-depth ophthalmological guidelines. The guidelines do not propose a method for quantifying corneal crystals during follow-up. Also, their annual follow-up schedule is, in our opinion, not suited for patients who have recently been diagnosed, because, in our opinion, they need to be seen on a more regular basis. A second article published by this group in 2016 [35] proposes recommendations to aid the transition of cystinosis patients from pediatric to adult care. In comparison to pediatrics, adult care patients are often seen by different professionals, primarily focused on their own specialty. This entails the risk of “fragmentation of care”, with possible negative repercussions on patients’ health. Regarding ophthalmological follow-up in our hospital, the same follow-up assessment is used in youngsters with cystinosis, seen by our pediatric ophthalmologist up until the age of 25, as in adult patients, seen by the anterior segment specialist, to provide a continuum of care.
Diagnosis, treatment and follow-up of patients with cystinosis present a number of challenges. Treatment is limited to eye drops or a gel solution, which are not always well-tolerated. Ophthalmological assessment of cystinosis patients is very complex. Researchers have agreed that an accurate measurement of the corneal crystal load is essential in assessing disease progression. The CCCS scale by Gahl and colleagues is widely used to assess the degree of crystal accumulation in patients with nephropathic cystinosis [2]. In clinical practice, the maximum score of 3.0 is quickly reached and does not allow the clinician to gauge the evolution of corneal crystals in more advanced cases. Therefore, the CCCS is, in our opinion, inadequate for follow-up ophthalmological examination of patients with cystinosis. AS-OCT is a non-contact imaging modality that may be an alternative to the CCCS score since it can provide an estimation of the depth of corneal crystal deposition. However, technical modalities that can quantify corneal crystals objectively are preferable. Among these, ICVM is the gold standard. However, its results are highly variable across technicians and the device is not widely available. Furthermore, the procedure represents a source of inconvenience to the patient. We recommend that confocal microscopy is performed at the second visit, after a healthy physician-patient relationship has been established and the patient has been informed about the procedure during the first appointment.
The rebound tonometer is easier to use in awake, young children but we prefer using the standard Goldmann tonometer as soon as the patient is old enough and consents to it [32].
Three population-based studies indicated that the risk of acute-closure glaucoma after dilation is extremely low [36]. In patients with narrow angles or those with obvious iris crystals [37], the risk of angle-closure glaucoma should be assessed by gonioscopy prior to installing dilating drops.
All patients receive post-dilation fundus photography by Optos, an ultra-widefield retinal single-capture imaging device that is generally well-tolerated, even by very photophobic patients. It allows us to examine the fundus even when strong photophobia is an issue.
In the long term, it is important to listen to patients and encourage them to continue the regular use of eye drops, inform them about progress in the field and assess the evolution of crystal accumulation and associated symptoms in order to adapt the treatment as much as possible. It should be noted that the standardized clinical ophthalmological assessment is a representation of how follow-up of cystinosis patients is conducted in our hospital. International guidelines remain to be addressed in a future publication. We plan to continue our multidisciplinary clinics and hope to be able to compare this work on a to be drafted consensus document in the future.
No previously published study has investigated the effectiveness of a multidisciplinary approach to the treatment of this condition compared with usual care, likely due to the rarity of cystinosis. However, several studies have been conducted to evaluate its effectiveness in other conditions. Two retrospective studies that focused on pediatric patients with chronic kidney disease compared clinical outcomes in those who were treated by a team of specialists and those who received regular care [38, 39] with significantly better results in the former group in both studies. A study conducted of high-risk elderly patients showed significant reduction in health care utilization and improvement in the quality of life after consultation with a multidisciplinary team of medical professionals [40]. However, although a multidisciplinary approach is effective, it may also result in higher treatment costs [41].
Conclusion
A number of devices and techniques can be used in ophthalmological assessment of patients with cystinosis. The standardized clinical ophthalmological assessment presented here represents a diagnostic, treatment and follow-up algorithm that is based on extensive clinical experience. In systemic diseases such as cystinosis, a special emphasis should be placed on a multidisciplinary approach to treatment in order to achieve the best clinical outcome.
References
Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347(2):111–21.
Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71(1–2):100–20.
Nesterova G, Gahl W. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr Nephrol. 2008;23(6):863–78.
Abderhalden E. Familiäre Cystindiathese. Hoppe-Seyler´ s Zeitschrift für Physiol Chemie. 1903;38(5–6):557–61.
Oshima RG, Willis RC, Furlong CE, Schneider JA. Binding assays for amino acids. The utilization of a cystine binding protein from Escherichia coli for the determination of acid-soluble cystine in small physiological samples. J Biol Chem. 1974;249(19):6033–9.
Jonas AJ, Smith ML, Schneider JA. ATP-dependent lysosomal cystine efflux is defective in cystinosis. J Biol Chem. 1982;257(22):13185–8.
Touchman JW, Anikster Y, Dietrich NL, Maduro VV, McDowell G, Shotelersuk V, et al. The genomic region encompassing the nephropathic cystinosis gene (CTNS): complete sequencing of a 200-kb segment and discovery of a novel gene within the common cystinosis-causing deletion. Genome Res. 2000;10(2):165–73.
Gahl WA, Thoene JG, Schneider JA. Cystinosis: a disorder of lysosomal membrane transport. In: Scriver C, Beaudet A, Sly W, et al., editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001.
Thoene JG, Oshima RG, Crawhall JC, Olson DL, Sghneder JA. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976;58(July):180–9.
Jézégou A, Llinares E, Anne C, Kieffer-Jaquinod S, O’Regan S, Aupetit J, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA. 2012;109(50):E3434–43.
Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147(4):242–50.
Tsilou E, Zhou M, Gahl W, Sieving PC, Chan C-C. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Surv Ophthalmol. 2007;52(1):97–105.
Bürki E. Über die Cystinkrankheit im Kleinkindesalter unter besonderer Berücksichtigung des Augenbefundes. Ophthalmologica. 1941;101(5):257–72.
Kaiser-Kupfer MI, Fujikawa L, Kuwabara T, Jain S, Gahl WA. Removal of corneal crystals by topical cysteamine in nephropathic cystinosis. N Engl J Med. 1987;316(13):775–9.
Wong VG. Ocular manifestations in cystinosis. Birth Defects Orig Artic Ser. 1976;12:181–6.
Labbe A, Niaudet P, Loirat C, Charbit M, Guest GBC. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis. Ophthalmology. 2009;116:870–6.
Liang H, Baudouin C, Tahiri R, Hassani J, Labbe A. Photophobia and corneal crystal density in nephropathic cystinosis: an in vivo confocal microscopy and anterior-segment optical coherence tomography study. Invest Ophthalmol Vis Sci. 2015;56:3218–25.
Weiss JS, Khemichian AJ. Differential diagnosis of Schnyder corneal dystrophy. Dev Ophthalmol. 2011;48:67–96.
Kleta R, Blair SC, Bernardini I, Kaiser-Kupfer MI, Gahl WA. Keratopathy of multiple myeloma masquerading as corneal crystals of ocular cystinosis. Mayo Clin Proc. 2004;79(3):410–2.
Dufier JL, Dhermy P, Gubler MC, Gagnadoux MF, Broyer M. Ocular changes in long-term evolution of infantile cystinosis. Ophthalmic Paediatr Genet. 1987;8(2):131–7.
Dureau P, Broyer M, Dufier JL. Evolution of ocular manifestations in nephropathic cystinosis: a long-term study of a population treated with cysteamine. J Pediatr Ophthalmol Strabismus. 2003;40(3):142–6.
Martín-Begué N, Alarcón S, Wolley-Dod C, Lara LE, Madrid Á, Cano P, et al. Intracranial hypertension in cystinosis is a challenge: experience in a children’s hospital. JIMD Rep. 2016. doi:10.1007/8904_2016_18.
Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986;104(5):706–11.
Bradbury JA, Danjoux JP, Voller J, Spencer M, Brocklebank T. A randomised placebo-controlled trial of topical cysteamine therapy in patients with nephropathic cystinosis. Eye. 1991;5:755–60.
Jones NP, Postlethwaite RJ, Noble JL. Clearance of corneal crystals in nephropathic cystinosis by topical cysteamine 0.5%. Br J Ophthalmol. 1991;75:311–2.
Kaiser-Kupfer MI, Gazzo MA, Datiles MB, Caruso RC, Kuehl EM, Gahl WA. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol. 1990;108(5):689–93.
Shams F, Livingstone I, Oladiwura D, Ramaesh K. Treatment of corneal cystine crystal accumulation in patients with cystinosis. Clin Ophthalmol. 2014;8:2077–84.
Iwata F, Kuehl EM, Reed GF, McCain LM, Gahl WA, Kaiser-Kupfer MI. A randomized clinical trial of topical cysteamine disulfide (cystamine) versus free thiol (cysteamine) in the treatment of corneal cystine crystals in cystinosis. Mol Genet Metab. 1998;64(4):237–42.
European Commission. Community register of medicinal products for human use. In: Cystadrops—Product information. Eur Comm [Internet]. 2017. http://ec.europa.eu/health/documents/community-register/html/h1049.htm. Accessed 22 Apr 2017.
Liang H, Labbe A, Le Mouhaer J, Plisson C, Baudouin C. A new viscous cysteamine eye drops treatment for ophthalmic cystinosis: an open-label randomized comparative phase III pivotal study. Invest Ophthalmol Vis Sci. 2017;58(4):2275–83.
Keklikci U, Soker SI, Sakalar YB, Unlu K, Ozekinci S, Tunik S. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn J Ophthalmol. 2008;52(5):357–62.
(NICE) NI for H and CE. Icare rebound tonometer to measure intraocular pressure. 2016;1–38. www.nice.org.uk/guidance/mib57. Accessed 22 Feb 2017.
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–50.
Ariceta G, Camacho JA, Fernandez-Obispo M, Fernandez-Polo A, Gamez J, Garcia-Villoria J, et al. Cystinosis in adult and adolescent patients: recommendations for the comprehensive care of cystinosis. Nefrologia. 2015;35(3):304–21.
Ariceta G, Antonio J, Fernández-obispo M, Fernández-polo A, Gámez J, García-villoria J, et al. Special article: a coordinated transition model for patients with cystinosis: from paediatrics to adult care. Nefrologia. 2016;6:1–15.
Liew G, Mitchell P, Wang JJ, Wong TY. Fundoscopy: to dilate or not to dilate? Vol. 332, BMJ (Clinical research ed.). England; 2006, p. 3.
Mungan N, Nischal KK, Heon E, MacKeen L, Balfe JW, Levin AV. Ultrasound biomicroscopy of the eye in cystinosis. Arch Ophthalmol (Chicago, Ill 1960). 2000;118(10):1329–33.
Ajarmeh S, Er L, Brin G, Djurdjev O, Dionne JM. The effect of a multidisciplinary care clinic on the outcomes in pediatric chronic kidney disease. Pediatr Nephrol. 2012;27(10):1921–7.
Menon S, Valentini RP, Kapur G, Layfield S, Mattoo TK. Effectiveness of a multidisciplinary clinic in managing children with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(7):1170–5.
Ritchie C, Andersen R, Eng J, Garrigues SK, Intinarelli G, Kao H, et al. Implementation of an interdisciplinary, team-based complex care support health care model at an academic medical center: impact on health care utilization and quality of life. PLoS One. 2016;11(2):e0148096.
Wijeysundera HC, Austin PC, Wang X, Bennell MC, Abrahamyan L, Ko DT, et al. The effect of multidisciplinary heart failure clinic characteristics on 1-year postdischarge health care costs: a population-based study. Med Care. 2014;52(3):272–9.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors would like to thank Cecile Duchesnes, PhD, and Georgii Filatov of Springer Healthcare Communications, who assisted in the drafting of this manuscript. This medical writing assistance was funded by Orphan Europe. The article processing charges were funded by Orphan Europe.
Disclosures
Pinxten AM, Hua MT, Simpson JL, Hohenfellner K, Levtchenko EN and Casteels I have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9B18F0605FE630F9.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pinxten, AM., Hua, MT., Simpson, J. et al. Clinical Practice: A Proposed Standardized Ophthalmological Assessment for Patients with Cystinosis. Ophthalmol Ther 6, 93–104 (2017). https://doi.org/10.1007/s40123-017-0089-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-017-0089-3