Abstract
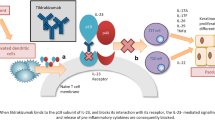
Adalimumab (Humira®) is a fully human monoclonal antibody against tumour necrosis factor (TNF), formulated for subcutaneous administration. It is well established in the treatment of adults with moderate-to-severe chronic plaque psoriasis and has recently received approval in the EU for the treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age. In a phase III trial in paediatric patients, a significantly greater proportion of patients receiving adalimumab 0.8 mg/kg (to a maximum of 40 mg) every other week (eow) achieved a ≥75 % improvement from baseline in Psoriasis Area and Severity Index than those receiving methotrexate after 16 weeks of treatment. In adults, well-designed randomized clinical trials demonstrated that adalimumab 40 mg eow effectively reduced the signs and symptoms of psoriasis and improved dermatology-specific and general measures of health-related quality of life, with these benefits sustained during long-term treatment. Adalimumab was generally well tolerated, compared with placebo or methotrexate, during clinical trials in paediatric and adult patients with chronic plaque psoriasis. Thus, adalimumab remains an important treatment strategy in adults with moderate-to-severe chronic plaque psoriasis and provides a promising new systemic treatment option for children and adolescents from 4 years of age with severe psoriasis.
Similar content being viewed by others
References
National Psoriasis Foundation. Psoriasis overview and psoriasis statistics. 2015. https://www.psoriasis.org/. Accessed 15 Oct 2015.
Bronckers IM, Paller AS, van Geel MJ, et al. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373–84.
Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–94.
NICE. Psoriasis: the assessment and management of psoriasis (NICE guidelines [CG153]). 2012. http://www.nice.org.uk/guidance/cg153. Accessed 15 Oct 2015.
European Dermatology Forum (EDF). European S3-guidelines on the systemic treatment of psoriasis vulgaris: update 2015. 2015. http://www.euroderm.org/edf/. Accessed 13 Oct 2015.
de Oliveira MF, de Rocha BO, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20.
European Medicines Agency. Humira pre-filled pen, pre-filled syringe and vial: summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 15 Oct 2015.
Croom KF, McCormack PL. Adalimumab: in plaque psoriasis. Am J Clin Dermatol. 2009;10(1):43–50.
Johnson-Huang LM, McNutt NS, Krueger JG, et al. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. J Clin Immunol. 2009;29(3):247–56.
Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60.
Gordon KB, Bonish BK, Patel T, et al. The tumour necrosis factor-alpha inhibitor adalimumab rapidly reverses the decrease in epidermal Langerhans cell density in psoriatic plaques. Br J Dermatol. 2005;153(5):945–53.
Marble DJ, Gordon KB, Nickoloff BJ. Targeting TNFalpha rapidly reduces density of dendritic cells and macrophages in psoriatic plaques with restoration of epidermal keratinocyte differentiation. J Dermatol Sci. 2007;48(2):87–101.
Hendriks AG, van der Velden HM, Wolberink EA, et al. The effect of adalimumab on key drivers in the pathogenesis of psoriasis. Br J Dermatol. 2014;170(3):571–80.
Soegaard-Madsen L, Johansen C, Iversen L, et al. Adalimumab therapy rapidly inhibits p38 mitogen-activated protein kinase activity in lesional psoriatic skin preceding clinical improvement. Br J Dermatol. 2010;162(6):1216–23.
Johansen C, Vinter H, Soegaard-Madsen L, et al. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. Br J Dermatol. 2010;163(6):1194–204.
Goldminz AM, Suarez-Farinas M, Wang AC, et al. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol. 2015;151(8):837–46.
Strober BE, Poulin Y, Teller C, et al. Changes in C-reactive protein in patients with moderate-to-severe psoriasis switched to adalimumab therapy after suboptimal response to etanercept, methotrexate or phototherapy. J Eur Acad Dermatol Venereol. 2014;28(12):1701–6.
Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15(1):45–50.
Bissonnette R, Tardif JC, Harel F, et al. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6(1):83–90.
Sharma S, Noertersheuser P, Mostafa N. Pharmacokinetics of adalimumab in adult patients with moderate to severe chronic plaque psoriasis [abstract no. P1662]. In: 23rd European Academy of Dermatology and Venereology (EADV) Congress. 2014.
Keizer RJ, Huitema AD, Schellens JH, et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Papp K, Thaci D, Marcoux D. Efficacy and safety of adalimumab versus methotrexate treatment in pediatric patients with severe chronic plaque psoriasis: results from the 16-week randomized, double-blind period of a phase 3 study [abstract plus poster]. In: The 23rd World Congress of Dermatology. 2015.
Philipp S, Ghislain P-D, Landells I. Efficacy, safety of adalimumab vs methotrexate in pediatric patients with severe chronic plaque psoriasis: results from the treatment withdrawal and double-blind retreatment periods of a phase 3 study [abstract plus poster]. In: The 23rd World Congress of Dermatology. 2015.
European Medicines Agency. Extension of indication variation assessment report: procedure no. EMEA/H/C/000481/II/0134 2015. http://www.ema.europa.eu/. Accessed 15 Oct 2015.
Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–15.
Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–66.
Kimball AB, Bensimon AG, Guerin A, et al. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trial. Am J Clin Dermatol. 2011;12(1):51–62.
Menter A, Gordon KB, Leonardi CL, et al. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol. 2010;63(3):448–56.
Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo-controlled trial in patients with psoriasis. J Drugs Dermatol. 2015;14(8):864–8.
Revicki DA, Willian MK, Menter A, et al. Impact of adalimumab treatment on patient-reported outcomes: results from a Phase III clinical trial in patients with moderate to severe plaque psoriasis. J Dermatolog Treat. 2007;18(6):341–50.
Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158(3):549–57.
Papp KA, Signorovitch J, Ramakrishnan K, et al. Effects of adalimumab versus placebo on risk of symptom worsening in psoriasis and subsequent impacts on health-related quality-of-life: analysis of pooled data from two randomized, double-blind, placebo-controlled, multicentre clinical trials. Clin Drug Investig. 2011;31(1):51–60.
Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol. 2012;66(2):241–51.
Papp K, Menter A, Poulin Y, et al. Long-term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open-label extension study. J Eur Acad Dermatol Venereol. 2013;27(5):634–42.
Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164(2):434–41.
Poulin Y, Sheth P, Gu Y, et al. Health-related quality of life worsens disproportionately to objective signs of psoriasis after withdrawal of adalimumab therapy. Dermatol Ther. 2014;4(1):33–42.
Gordon KB, Gottlieb AB, Langely RG, et al. Adalimumab retreatment successfully restores clinical response and health-related quality of life in patients with moderate to severe psoriasis who undergo therapy interruption. J Eur Acad Dermatol Venereol. 2015;29(4):767–76.
Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163(2):402–11.
Leonardi C, Langley RG, Papp K, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011;147(4):429–36.
Leonardi C, Papp K, Strober B, et al. The long-term safety of adalimumab treatment in moderate to severe psoriasis: a comprehensive analysis of all adalimumab exposure in all clinical trials. Am J Clin Dermatol. 2011;12(5):321–37.
Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–24.
Menter A, Thaci D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73(3):410–9 (e6).
Hsu L, Snodgrass BT, Armstrong AW. Antidrug antibodies in psoriasis: a systematic review. Br J Dermatol. 2014;170(2):261–73.
Menting SP, van Lumig PP, de Vries AC, et al. Extent and consequences of antibody formation against adalimumab in patients with psoriasis: one-year follow-up. JAMA Dermatol. 2014;150(2):130–6.
Asahina A, Nakagawa H, Etoh T. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310.
Asahina A, Ohtsuki M, Etoh T, et al. Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese study. J Dermatol. 2015;14(11):1042–52.
Lebrec H, Ponce R, Preston BD, et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin. 2015;31(3):557–74.
Farhangian ME, Feldman SR. Immunogenicity of biologic treatments for psoriasis: therapeutic consequences and the potential value of concomitant methotrexate. Am J Clin Dermatol. 2015;16(4):285–94.
Lecluse LL, Driessen RJ, Spuls PI, et al. Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol. 2010;146(2):127–32.
Chiu HY, Wang TS, Chan CC, et al. Risk factor analysis for the immunogenicity of adalimumab associated with decreased clinical response in Chinese patients with psoriasis. Acta Derm Venereol. 2015;95(6):711–6.
Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28(4):438–53.
Bissonnette R, Bolduc C, Poulin Y, et al. Efficacy and safety of adalimumab in patients with plaque psoriasis who have shown an unsatisfactory response to etanercept. J Am Acad Dermatol. 2010;63(2):228–34.
Strober BE, Poulin Y, Kerdel FA, et al. Switching to adalimumab for psoriasis patients with a suboptimal response to etanercept, methotrexate, or phototherapy: efficacy and safety results from an open-label study. J Am Acad Dermatol. 2011;64(4):671–81.
Sator P, Richter L, Saxinger W, et al. Adalimumab in the treatment of moderate-to-severe chronic plaque psoriasis in patients switching from other biologics. J Eur Acad Dermatol Venereol. 2015;29(9):1742–9.
Gniadecki R, Bang B, Bryld LE, et al. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–52.
Warren RB, Smith CH, Yiu ZZ, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–40.
van den Reek JM, Zweegers J, Kievit W, et al. ‘Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol. 2014;171(5):1189–96.
van den Reek JM, Tummers M, Zweegers J, et al. Predictors of adalimumab drug survival in psoriasis differ by reason for discontinuation: long-term results from the Bio-CAPTURE registry. J Eur Acad Dermatol Venereol. 2015;29(3):560–5.
Esposito M, Gisondi P, Cassano N, et al. Survival rate of antitumour necrosis factor-alpha treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br J Dermatol. 2013;169(3):666–72.
Inzinger M, Wippel-Slupetzky K, Weger W, et al. Survival and effectiveness of tumour necrosis factor-alpha inhibitors in the treatment of plaque psoriasis under daily life conditions: report from the psoriasis registry Austria. Acta Derm Venereol. 2015. doi:10.2340/00015555-2214.
Mustonen A, Mattila K, Leino M, et al. Psoriasis causes significant economic burden to patients. Dermatol Ther (Heidelb). 2014;4(1):115–24.
Fragoulakis V, Raptis E, Vitsou E, et al. Annual biologic treatment cost for new and existing patients with moderate to severe plaque psoriasis in Greece. Clinicoecon Outcomes Res. 2015;7:73–83.
Zhang W, Islam N, Ma C, et al. Systematic review of cost-effectiveness analyses of treatments for psoriasis. Pharmacoeconomics. 2015;33(4):327–40.
Ruano J, Isla-Tejera B, Jimenez-Puya R, et al. Long-term cost-effectiveness analysis of etanercept and adalimumab for plaque psoriasis not associated with arthritis. Dermatol Ther (Heidelb). 2013;3(2):131–42.
D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72(4):589–98.
Sawyer LM, Wonderling D, Jackson K, et al. Biological therapies for the treatment of severe psoriasis in patients with previous exposure to biological therapy: a cost-effectiveness analysis. Pharmacoeconomics. 2015;33(2):163–77.
Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
Ahn CS, Gustafson CJ, Sandoval LF, et al. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013;14(4):315–26.
Ferrandiz C, Garcia A, Blasco AJ, et al. Cost-efficacy of adalimumab, etanercept, infliximab and ustekinumab for moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2012;26(6):768–77.
Puig L, Lopez A, Vilarrasa E, et al. Efficacy of biologics in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials with different time points. J Eur Acad Dermatol Venereol. 2014;28(12):1633–53.
Galvan-Banqueri M, Marin Gil R, Santos Ramos B, et al. Biological treatments for moderate-to-severe psoriasis: indirect comparison. J Clin Pharm Ther. 2013;38(2):121–30.
Reich K, Burden AD, Eaton JN, et al. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol. 2012;166(1):179–88.
Messori A, Fadda V, Maratea D, et al. Biological drugs for the treatment of moderate-to-severe psoriasis by subcutaneous route: determining statistical equivalence according to evidence-based methods. Clin Drug Investig. 2014;34(8):593–8.
Puig L. On clinical thresholds, clinical equivalents and indirect comparisons of biological treatments for moderate-to-severe psoriasis. J Clin Pharm Ther. 2015;40(2):131–4.
Feldman SR. Factors for choosing the right biologic treatment. The Dermatologist. 2014;22(9). http://www.the-dermatologist.com/content/factors-choosing-right-biologic-treatment. Accessed 13 Jul 2015.
Acknowledgments
During the peer review process, the manufacturer of the adalimumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Celeste Burness and Kate McKeage are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: C. Blandizzi, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy; S. Mantarro Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy; A.G. Richetta, Clinic of Dermatology, Department of Internal Medicine and Medical Specialties, “Sapienza” University of Rome, Rome, Italy; P. - G. Sator, Department of Dermatology, Hospital Hietzing, Vienna, Austria; R. B. Vender, Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Rights and permissions
About this article
Cite this article
Burness, C.B., McKeage, K. Adalimumab: A Review in Chronic Plaque Psoriasis. Drugs 75, 2119–2130 (2015). https://doi.org/10.1007/s40265-015-0503-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0503-x