Abstract
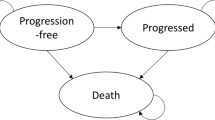
The National Institute for Health and Care Excellence (NICE) invited the manufacturer of azacitidine (Celgene) to submit evidence for the clinical and cost effectiveness of this drug for the treatment of acute myeloid leukaemia with more than 30 % bone marrow blasts in adults who are not eligible for haematopoietic stem cell transplantation, as part of the NICE’s Single Technology Appraisal process. The Peninsula Technology Assessment Group was commissioned to act as the Evidence Review Group (ERG). The ERG produced a critical review of the evidence contained within the company’s submission to NICE. The clinical effectiveness data used in the company’s economic analysis were derived from a single randomised controlled trial, AZA-AML-001. It was an international, multicentre, controlled, phase III study with an open-label, parallel-group design conducted to determine the efficacy and safety of azacitidine against a conventional care regimen (CCR). The CCR was a composite comparator of acute myeloid leukaemia treatments currently available in the National Health Service: intensive chemotherapy followed by best supportive care (BSC) upon disease relapse or progression, non-intensive chemotherapy followed by BSC and BSC only. In AZA-AML-001, the primary endpoint was overall survival. Azacitidine appeared to be superior to the CCR, with median overall survival of 10.4 and 6.5 months, respectively. However, in the intention-to-treat analysis, the survival advantage associated with azacitidine was not statistically significant. The company submitted a de novo economic evaluation based on a partitioned survival model with four health states: “Remission”, “Non-remission”, “Relapse/Progressive disease” and “Death”. The model time horizon was 10 years. The perspective was the National Health Service and Personal Social Services. Costs and health effects were discounted at the rate of 3.5 % per year. The base-case incremental cost-effectiveness ratio (ICER) of azacitidine compared with the CCR was £20,648 per quality-adjusted life-year (QALY) gained. In the probabilistic sensitivity analysis, the mean ICER was £17,423 per QALY. At the willingness-to-pay of £20,000, £30,000 and £50,000 per QALY, the probability of azacitidine being cost effective was 0.699, 0.908 and 0.996, respectively. The ERG identified a number of errors in Celgene’s model and concluded that the results of the company’s economic evaluation could not be considered robust. After amendments to Celgene’s model, the base-case ICER was £273,308 per QALY gained. In the probabilistic sensitivity analysis, the mean ICER was £277,123 per QALY. At a willingness-to-pay of £100,000 per QALY, the probability of azacitidine being cost effective was less than 5 %. In all exploratory analyses conducted by the ERG, the ICER exceeded the NICE’s cost-effectiveness threshold range of £20,000–30,000 per QALY. Given the evidence provided in the submission, azacitidine did not fulfil NICE’s end-of-life criteria. After considering the analyses performed by the ERG and submissions from clinician and patient experts, the NICE Appraisal Committee did not recommend azacitidine for this indication.

Source: individual patient data from AZA-AML-001 trial, reproduced with permission from the submission


Source: the ERG’s report [22]

Source: the ERG’s report [22]
Similar content being viewed by others
References
National Institute For Health and Care Excellence. NICE Charter. 2013. https://www.nice.org.uk/Media/Default/About/Who-we-are/NICE_Charter.pdf. Accessed 13 July 2016.
National Institute for Health and Care Excellence. Guide to the single technology appraisal process. 2009. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/Guide-to-the-single-technology-appraisal-process.pdf. Accessed 6 April 2016.
National Institute for Health and Care Excellence. Azacitidine for treating acute myeloid leukaemia with more than 30 % bone marrow blasts. NICE technology appraisal guidance [TA399]. 2016. https://www.nice.org.uk/guidance/ta399. Accessed 29 July 2016.
National Health Service. Acute myeloid leukaemia. 2016. http://www.nhs.uk/conditions/Leukaemia-acute/Pages/Introduction.aspx. Accessed 6 April 2016.
Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;. doi:10.1177/1947601911408076.
Cancer Research UK. Acute myeloid leukaemia (AML) incidence statistics. 2011–2013. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-aml/incidence. Accessed 6 April 2016.
Epidemiology and Cancer Statistics Group at the University of York DoHS, The University of York. Haematological Malignancy Research Network. Incidence. https://www.hmrn.org/statistics/incidence. Accessed 6 April 2016.
National Cancer Institute Surveillance, Epidemiology, and End Results Program. Acute myeloid leukemia, 5-year relative survival (percent) 2003–2009 by age at diagnosis. http://seer.cancer.gov/archive/csr/1975_2010/browse_csr.php?sectionSEL=13&pageSEL=sect_13_table.16.html#table3. Accessed 6 April 2016.
Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;. doi:10.1200/JCO.2012.43.4738.
Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;. doi:10.3324/haematol.2012.066100.
Appelbaum F. The use of bone marrow and peripheral blood stem cell transplantation in the treatment of cancer. Cancer J Clin. 1996;46(3):142–64.
Doehner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;. doi:10.1182/blood-2009-07-235358.
Milligan DW, Grimwade D, Cullis JO, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135(4):450–74.
NICE. Azacitidine for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia. 2011. https://www.nice.org.uk/guidance/ta218. Accessed 6 April 2016.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. http://publications.nice.org.uk/pmg9. Accessed 6 April 2016.
Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30 % blasts. Blood. 2015;. doi:10.1182/blood-2015-01-621664.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–88.
Robins JM, Tsiatis AA. Correcting for non-compliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory. 1991;20(8):2609–31.
Branson M, Whitehead J. Estimating a treatment effect in survival studies in which patients switch treatment. Stat Med. 2002;21(17):2449–63.
Latimer NR, Abrams KR. NICE DSU Technical Support Document 16: adjusting survival time estimates in the presence of treatment switching. 2014. http://www.nicedsu.org.uk. Accessed 13 July 2016.
Mujica Mota R, Varley-Campbell J, Tikhonova I, et al. Azacitidine for treating acute myeloid leukaemia with more than 30 % bone marrow blasts: a single technology appraisal. 2016. https://www.nice.org.uk/guidance/GID-TA10000/documents/committee-papers. Accessed 22 Sept 2016.
NICE. NICE DSU Technical Support Document 14: survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data. 2013. http://www.nicedsu.org.uk/NICE%20DSU%20TSD%20Survival%20analysis.updated%20March%202013.v2.pdf. Accessed 7 April 2016.
Joint Formulary Committee. British National Formulary 69. The authority on the selection and use of medicines. 2015. p. 588.
Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit; 2014.
Department of Health. NHS reference costs 2013 to 2014. London: NHS; 2014.
National Institute for Health Research. Health Technology Assessment (HTA) Programme. 2016. http://www.hta.ac.uk. Accessed 8 April 2016.
Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Pharmacoeconomics. 2013;31:361. doi:10.1007/s40273-013-0032-y.
Acknowledgments
The authors thank Dr. Mary Bond for commenting on the critique of the company’s clinical evidence. The authors also acknowledge the excellent administrative support of Mrs. Sue Whiffin and Ms. Jenny Lowe. This summary has not been externally reviewed by PharmacoEconomics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (project number 15/64/10). Visit the HTA programme website for further information [27]. The summary of the ERG’s report was compiled after NICE issued the Final Appraisal Determination. This report presents independent research commissioned by the NIHR. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, CCF, NETSCC, the NIHR HTA Programme or the Department of Health.
Conflict of interest
I. T., M. H., T. S., C. C., J. V.-C. C. R. and R. M. M. declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40273_2016_453_MOESM10_ESM.docx
Supplementary material 10 (DOCX 33 kb)
CHEERS checklist Table 8 (Online Resource) contains the CHEERS checklist [28], which gives references to where in the manuscript or Online Resource the CHEERS checklist items are satisfied.
Rights and permissions
About this article
Cite this article
Tikhonova, I.A., Hoyle, M.W., Snowsill, T.M. et al. Azacitidine for Treating Acute Myeloid Leukaemia with More Than 30 % Bone Marrow Blasts: An Evidence Review Group Perspective of a National Institute for Health and Care Excellence Single Technology Appraisal. PharmacoEconomics 35, 363–373 (2017). https://doi.org/10.1007/s40273-016-0453-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0453-5