Abstract
In oncology, diagnostic imaging plays a major role in staging, therapy assessment and in the evaluation of tumor biology. Multimodality imaging, and more specifically positron emission tomography/computed tomography (PET/CT), has matured into an important imaging tool. The recent introduction of the integrated whole-body PET/magnetic resonance imaging (MRI) represents the addition of a promising methodology in clinical practice. Combining the metabolic data of PET with the anatomical and functional information provided by MRI and fMRI may further improve the diagnostic value of each method alone. Although the literature is still limited, data indicate a potential advantage of PET/MRI over PET/CT in all the indications where MRI is superior to CT, as well as in the evaluation of tumor biology. Integrated PET/MRI might be performed in addition to the existing imaging modality in specific regions. Moreover, integrated PET/MRI is an alternative to PET/CT when a low radiation dose is required, i.e. in children and in repeated imaging. In the rapidly evolving field of diagnostic imaging, the role of a new modality should be accurately evaluated. Further studies are needed to test the diagnostic accuracy of PET/MRI in different oncology indications. Whether PET/MRI will replace PET/CT or be a complementary methodology and whether it represents true diagnostic progress remains to be evaluated, also taking into account economic considerations.
Similar content being viewed by others
Introduction
Morphological and molecular imaging, such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET), play a key role in clinical oncology: diagnosis as well as staging and treatment evaluation rely on imaging findings. However, each of these imaging procedures suffers from well-known inherent limitations. The objective of combining anatomical and functional information into a single image has long been pursued. In the wake of research efforts focusing on software coregistration, the introduction of hybrid PET/CT systems [1] substantially changed cancer imaging. Since the introduction of commercial PET/CT scanners, both PET and CT technology have continued to evolve, improving image quality [2–4]. 2-(18F)-fluoro-2-deoxy-d-glucose (FDG) PET/CT, and to a lesser extent other radiotracers, have had a major impact on oncology over the past decade, improving clinical management [5–10]. Despite the success of PET/CT, the possibility of replacing CT with MRI has been increasingly investigated [11–13], and was actually investigated even before the introduction of PET/CT [14]. These efforts are driven by a series of considerations:
-
the possibility of lower patient exposure to radiation, given that MRI does not use ionizing radiation; this aspect is important in pediatric patients as well as in other situations such as cases requiring repeated studies;
-
MRI provides high-resolution anatomical and structural images offering better soft-tissue contrast resolution and a large variety of tissue contrasts, compared with CT;
-
MRI, through functional MRI (fMRI) and MR spectroscopy (MRS), provides a wealth of additional information able to enhance the diagnostic performance and quantitative capabilities of PET and help improve patient management and understanding of tumor biology.
On the other hand, MRI has several disadvantages:
-
examination time can be substantially longer than with CT, depending on the imaging protocol;
-
there still exist contraindications related to metal implants and pacemakers; metallic implants produce a local signal loss in MRI images, misleading the image segmentation procedure and resulting in classification of the region as air instead of tissue in the MRI-based attenuation map; this may result in underestimation of uptake;
-
a relatively low sensitivity for lung lesions, although new scanning parameters have dealt with this limitation; moreover, one or both lungs are occasionally not identified correctly and instead interpreted as air, thus leading to undercorrection of standardized uptake values in this area;
-
MRI is more expensive than CT.
In contrast to CT, MRI offers a multitude of “endogenous” contrasts and a high capability for soft-tissue differentiation, as well as many exogenous contrast media ranging from gadolinium-based agents to highly specific cellular markers. Having sensitivity in the picomolar range, PET is ideally suited for the visualization of specific molecules in living organisms. However, PET lacks the spatial resolution offered by MRI, which in turn lacks sensitivity. Therefore, the combination of PET and MRI is highly complementary. Unlike CT, MRI results in no additional radiation. Furthermore, as mentioned previously, MRI has interesting functional imaging capabilities, such as perfusion, BOLD effect, diffusion, and spectroscopy.
However, MRI cannot simply replace the CT component of a PET/CT scanner, since a fully integrated PET/MRI combination requires technological modifications of both the PET and the MRI. Indeed, MRI interferes with state-of-the art PET technology (i.e., PMT) and PET interferes with field gradients or MR radio frequency. The physics of and technical solutions to these problems are beyond the scope of this review and many excellent papers dealing with these issues have been published [15–18]. Moreover, practical PET/MRI implementations should also take in account the issue of accurate MRI-based methods for attenuation correction (AC) of the measured PET emission data, which is particularly important for quantitative PET. Whereas CT measures the attenuation coefficient of tissues at X-ray energies, the MRI signal is determined by tissue hydrogen density and relaxation properties, and thus, the derivation of 511-keV-photon attenuation coefficients is much more complex than in CT AC. Different methods for deriving attenuation maps from MR have been proposed [19–23].
Following the installation of the first head-only PET/MRI scanners [24], whole-body PET/MRI scanners have now been introduced in the market for clinical use. Taking into account the technological challenges inherent in combining PET and MRI, the three major companies (Siemens Healthcare, Philips Healthcare, and GE Healthcare) have proposed different PET/MRI designs.
As installation of the first PET/MRI systems is ongoing and only a few are already operating, the challenge is to understand the clinical potential of this new modality. Although the published data on integrated PET/MRI is still limited, first experiences with this imaging technique consistently indicate a better performance in those indications requiring high soft-tissue contrast. Furthermore, the true value of this new hybrid imaging modality lies in the simultaneous acquisition of fMRI data and metabolic PET information.
The aim of this review is to underline the oncological clinical applications of PET/MRI on the basis of published data on hybrid PET/MRI, when available, or on PET/CT and MRI. The current status of and clinical indications for PET/MRI in oncology will be discussed. A first section of the paper deals with whole-body FDG PET/MRI in cancer staging (TNM) and therapy response assessment. Thereafter, the focus shifts to non-FDG tracers and evaluation of tumor biology. Finally, a short paragraph is devoted to several methodological considerations.
Whole-body FDG PET/MRI
T staging
FDG PET/CT has been shown to be capable of accurately determining T stage in a few indications, such as head and neck tumors, non-small lung cancer and colorectal cancer [25–28]. However, since the evaluation of local tumor extent relies mainly on morphological data because of high spatial resolution, and the functional component of hybrid imaging does not add information on T stage, PET/MRI could prove to be superior to PET/CT in those indications in which MRI has been found to be more accurate than CT. On this basis, it can be anticipated that PET/MRI would be suitable in all those indications in which the high tissue contrast of MRI would allow higher accuracy: breast, head and neck, liver, musculoskeletal, and brain tumors.
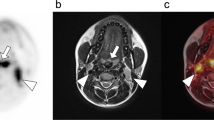
PET/MRI performed better than PET/CT in head and neck cancer [29], but did not add significant information to MRI alone in either simultaneous or fused imaging [29, 30]. In our own unpublished experience, MR/PET showed better soft-tissue contrast resolution (Fig. 1).
A 67-year-old male patient with squamous cell carcinoma of the upper alveolar ridge. From left to right, upper row: T1 TSE tra FAT SAT (after contrast), T2 TSE tra and PET/MRI fusion (FDG); lower row: DWI (b = 800), CT and CT (bone window). PET/MRI allows better lesion identification and anatomical location compared with PET/CT. In addition bone marrow involvement was found allowing correct T3 staging
In breast cancer, MR mammography shows high sensitivity and relatively low specificity, while FDG PET/CT is more specific and less sensitive [31, 32]. An increase in specificity from 53 to 97 % was observed by adding FDG and MRI data [33]. However, a study with software-based fusion of images did not show any significant benefit [34]. This discrepancy could be due to lesion dimensions, since small lesions are known to show poor FDG uptake [32].
FDG PET/CT has a low sensitivity in hepatic primary tumors (hepatocellular cancer), with radiotracers other than FDG showing better results [35]. No data on PET/MRI in this setting have been published so far; however, it is conceivable that combined PET/MRI, with the addition of fMRI, would perform as well as MRI alone.
T staging of colorectal cancer with software-based PET/MRI fusion is inaccurate [36], and it is improbable that a hybrid PET/MRI system would perform better than the MRI alone or even FDG PET/CT [28].
Although MRI, including fMRI, is the method of choice in T staging of primary bone tumors [37], high accuracy using FDG PET/CT has been reported [38]. The high soft-tissue contrast of MRI has made it the preferred modality in soft-tissue sarcomas with no advantage derived from adding FDG data [39, 40]. However, in both instances, i.e., the primary bone tumors and soft-tissue sarcomas, FDG PET/MRI, providing FDG uptake information, may be used to guide biopsies and in preoperative and radiation treatment planning [41].
The literature on FDG PET/CT in the staging of both Hodgkin and non-Hodgkin lymphomas is extensive, showing very high accuracy, which has led to a widespread use of this methodology [42–44]. Diffusion-weighted whole-body MRI has been proposed as an alternative to FDG PET/CT, showing comparable accuracy [45, 46]. However, more recent data suggest a more conservative approach, since overstaging was observed [47]. PET/MRI could be an alternative to PET/CT in lymphoma, particularly in younger patients, although no published data are available to date.
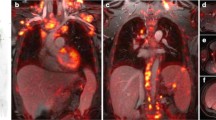
MRI offers an alternative morphological imaging approach avoiding additional radiation exposure in pediatric oncology patients. Thus, in children, PET/MRI can add significant information to that obtained through the use of separate imaging modalities. Data on the diagnostic value of combined and registered image analysis of FDG PET and MRI for staging and re-staging in pediatric oncology have recently been published [48]. On a lesion-based analysis, PET/MRI performed significantly better than the two imaging modalities alone [48]. Moreover, in our experience, appropriate MRI scanning parameters allow PET/MRI imaging of the lung (Fig. 2).
N staging
FDG PET/CT is more accurate than CT alone in the assessment of N stage in different malignant diseases, and shows significantly higher accuracy than whole-body MRI in staging lymph node metastatic spread [49, 50]. This advantage of FDG PET/CT over MRI and CT is due to the metabolic data: PET has the capability to detect viable tumor tissue in metastatic lymph nodes independently of their size. Instead, MRI relies on size-based lymph node assessment in N staging. However, both benign and malignant lymph nodes vary significantly in size: nodes larger than 1 can be benign, just as those smaller than 1 cm can be malignant. Therefore, PET/MRI is expected to perform as well as PET/CT, or even better by adding fMRI data. The major limitation would be, as in PET/CT, identification of small metastases or micrometastases because of spatial resolution. Available data from the literature are still limited, but initial reports are encouraging. A higher accuracy of PET/MRI compared with PET/CT has been observed in head and neck squamous cell cancer, although 30 % of patients were understaged [30]. Figure 3 shows a patient from our experience. No major advantage of PET/MRI over MRI alone would be expected in lymph node staging in breast cancer, where the clinical scenario must be established also taking into account other modalities such as sentinel lymph node biopsy. FDG PET/CT has been reported to show an accuracy of 80 % in N staging of colorectal cancer [28], comparable to MRI [51], while combined FDG PET/CT and MRI showed higher values [52]. FDG PET/CT has proven to be superior to all conventional imaging modalities, including MRI, in soft-tissue sarcomas [53], and again PET/MRI can be expected to perform at least as well, or better by integrating fMRI data. Analogously to breast cancer, the sentinel lymph node plays a major role in melanoma depending on tumor thickness. However, when the lymph node biopsy is positive imaging is recommended [54], and integrated PET/MRI will surely be an invaluable tool in melanoma patients.
A 72-year-old female patient with squamous cell carcinoma of the tongue base and lymph node involvement. From left to right, top row: STIR coronal (transaxial view), PET/MRI fusion, PET; second row: DWI (b = 800), ADC map, CT; third row: CT, STIR coronal, DWI (b = 800); bottom row: STIR coronal, PET/MRI fusion. PET/MRI characterized both lesion extent and nodal involvement better than CT/PET did
M staging
Some studies have compared FDG PET/CT with whole-body MRI for evaluation of distant metastases [6, 50, 55–57]. Results are different in different anatomical districts, with PET/CT found to be more accurate in assessing lung metastases and MRI in liver, bone, and obviously, brain metastases. However, certain sequences (single-shot, turbo spin echo) as well as diffusion-weighted imaging can lead to improvement in lung metastasis detection [58, 59]. PET/MRI might be expected to perform better than MRI in the same regions. Liver MRI, especially with the use of specific contrast agents, is remarkably more accurate than FDG PET/CT in detecting hepatic metastases [60]. The sensitivity of Gd-EOB-DTPA-enhanced MRI and retrospectively fused PET/MRI in the detection of liver metastases has been shown to be higher than that of PET/CT, while PET/MRI resulted in a non-significant increase in sensitivity when compared to contrast-enhanced MRI [61]. Although the first available clinical application of integrated PET/MRI was the brain, initial studies focused on primary brain tumors [24, 29]. Data from brain evaluation using whole-body MRI protocols demonstrated higher accuracy, compared with FDG PET/CT, in the detection of cerebral metastases [58]. However, whether the diagnostic performance of integrated FDG PET/MRI would be of clinical relevance is difficult to predict from the available information. Actually, different acquisition procedures could potentially benefit PET/MRI: dual-phase FDG imaging, dynamic contrast-enhanced MRI, and spectroscopy. Detection of bone metastases is based on imaging: X-ray skeletal survey, bone scintigraphy, CT, MRI and PET/CT. In a meta-analysis comparing data from more than 15,000 patients, MRI and FDG PET/CT were found to be equally accurate [62]. Different results have been found in studies comparing the two imaging modalities in different cancer types, with MRI showing better sensitivity in the detection of bone metastases in breast cancer and lower sensitivity in lung cancer patients [63, 64]. Moreover, diffuse bone marrow infiltration is difficult to detect on PET/CT [57], but more reliably detected on MRI. Thus, for detecting bone metastases, integrated PET/MRI has the potential to overcome the limitations of each modality and become the procedure of choice.
Therapy response assessment
Although therapy response in solid tumors has usually been based solely on systematic assessments of tumor size, using, for example, the WHO, RECIST, and International Workshop Criteria (IWC) for lymphoma [65–67], these methods do have well-recognized limitations. For this reason, there has been a considerable and growing interest in molecular imaging techniques. FDG PET/CT has been shown to improve response assessment in several tumor types ranging from malignant lymphoma to a variety of solid tumors [67, 68]. In recent years, clinical studies have demonstrated that mid-treatment diffusion-weighted imaging could be used as an imaging response biomarker [69–72]. In the context of integrated PET/MRI, the addition of fMRI would enhance the performance of both modalities in the evaluation of both treatment response and recurrences.
A first prospective study of PET/MRI in therapy response assessment of head and neck tumors showed excellent results, combining the high negative predictive value of PET with the high sensitivity of MRI [30] (Fig. 4). In breast cancer, MRI and fMRI have proven to be sensitive to early therapy response [73], and FDG PET/CT is capable of predicting early response to neoadjuvant therapy [74]. The potential of integrated PET/MRI must be fully explored, since this hybrid methodology will likely play a key role in evaluating recurrence and therapy response in breast cancer patients. Both in chemoembolization and in radio frequency ablation of liver tumors, both primary and metastatic, FDG PET/CT and MRI (with the addition of fMRI) demonstrated better performances than CT, with high accuracy [75, 76]. Similar results have been obtained in the evaluation of neoadjuvant chemoradiotherapy in colorectal cancer [77, 78]. Although FDG PET/CT monitoring of therapy of lymphomas (Hodgkin and diffuse large B cell) has been included in the guidelines [42], more recent studies reported low and maybe suboptimal predictive value [79, 80] in early therapy assessment, probably related to therapy schemes different from classic chemotherapy, with higher predictive value in end-therapy evaluation. In this setting, MRI with the addition of fMRI (especially diffusion-weighted imaging) showed excellent and complementary results [81]. Additional advantages to be derived from integrated PET/MRI in the therapy response assessment of lymphomas would be the possibility of differentiating recurrence from thymic rebound by the use of chemical shift MRI [82] and the possibility of using the modality in children, thanks to the reduction of radiation exposure as compared with PET/CT. Figure 5 shows a patient with Hodgkin lymphoma from our experience.
A 47-year-old male patient with squamous cell carcinoma of the rhinopharynx treated with chemotherapy, radiotherapy and right latero-cervical lymphadenectomy. From left to right, top row: T1 TSE FAT SAT tra (post contrast), PET/MRI fusion, PET; middle row: ADC map, DWI (b = 800), CT; bottom row (coronal view): T1 TSE FAT SAT (after contrast), PET/MRI fusion, DWI (b = 800), PET. PET/MRI leads to correct identification of nodal relapse
Non-FDG tracers
FDG is utilized in more than 90 % of cancers in staging, re-staging, assessing therapy response and during the follow-up. However, not all tumors show a significant increase of metabolic activity on FDG imaging. Non-FDG tracers already used for clinical applications are 11C- and 18F-choline, 11C-methionine and 18F-FET, 18F-DOPA, 68Ga-DOTA-somatostatine analogs, 11C-acetate, 18F-FLT, and 124I (Table I). Imaging of intracranial masses was performed on a hybrid PET/MRI system capable of simultaneous PET/MRI acquisition administering 11C-methionine in patients with gliomas and 68 Ga-DOTA-somatostatine analogs in those with meningiomas [83]. Similar diagnostic image quality on the hybrid PET/MRI and the PET/CT studies was found, with excellent agreement between semi-quantitative data (i.e., the tumor-to-reference tissue ratios) of the PET/MRI and PET/CT systems (r = 0.98) [82]. 18F-FDOPA PET/MRI fusion provides precise anatomical localization of tracer uptake in patients with gliomas, with PET data capable of identifying tumors not visible on MRI [84]. In high-risk differentiated thyroid cancer patients, fused 124-iodine PET/MRI has proven to be superior to 124-iodine PET/CT in detecting lesions, particularly those <10 mm [85]. Both modalities were performed after thyroidectomy but before radioiodine therapy, and data provided by fused PET/MRI imaging provided more precise and tailored dosimetry [85]. MRI and fMRI (spectroscopy) is used to localize, stage, and obtain functional information in prostate cancer patients [86]. On the other hand, current evidence indicates that PET/CT with 11C-acetate and 11C-choline or 18F-fluorocholine might be useful in the diagnosis and staging of primary tumors, in guiding tumor biopsy, in detection of metastatic disease, in monitoring of response to therapies, and in prognostication [87]. Integrated PET/MRI imaging would be of clinical benefit by adding the diagnostic capabilities of each modality. Parametric fusion PET/MRI based on 11C-choline PET/CT and diffusion-weighted MRI for the identification of primary prostate cancer improves identification when compared to each modality alone [88]. A significant increase in accuracy, mainly due to a remarkable improvement in specificity, was found when fused 11C-acetate PET/MRI was compared with the performance of each individual modality alone in the detection of prostate cancer [89].
Although the majority of data published so far deals with fusion PET/MRI, there is an increasing evidence that integration of the two modalities would yield better performances in the setting of non-FDG tracers.
Tumor biology
Both in the preclinical and in clinical oncology applications there is a growing need for in vivo detection techniques to obtain better characterization of cellular and subcellular processes. Molecular imaging as stand-alone or multimodality methods has become an important discipline. Among the latter PET/CT has emerged as a successful imaging method and the recent introduction of PET/MRI will pave the way for a better understanding of oncologic processes, in both the clinical and preclinical settings. PET provides information on metabolic and molecular parameters with high sensitivity of data at molecular level, but limitations as regards tissue morphology. On the other hand, MRI provides a variety of data from the anatomical to the metabolic level, the latter with lower sensitivity at the molecular level but to a large extent synergic to those of PET. Several aspects of the biology of cancer might be evaluated with integrated PET/MRI: from metabolism to angiogenesis, from proliferation to apoptosis, from stem cells to metastases. Each of these could provide further insights into the biology and pathophysiology of cancer. More importantly from a clinical point of view, molecular imaging can be efficiently used as imaging surrogate in monitoring therapies and in prognosis evaluation. At present, only PET is able to evaluate cellular metabolism and proliferation in vivo, by using appropriate tracers such as FDG, l-DOPA, methionine analogs, and FLT (Table 1) [90–92]. Both PET, with 18F-fluoromisonidazole, and MRI, with spectroscopy and BOLD fMRI, can be used to study hypoxia [90–92]. Data on apoptosis can be obtained from PET imaging with radiolabeled annexin or indirectly from diffusion-weighted MRI [90–92]. Dynamic contrast-enhanced MRI and PET with perfusion tracer are used to assess the tumor perfusion. In addition, molecular markers such as integrins, VEGF and its receptors, and metalloproteinase can be evaluated using appropriate labeled radiotracers or MRI with paramagnetic nanoparticles [90–92].
PET/MRI, in which each modality provides supplementary information to that provided by the other, is destined to play a major role as a surrogate-imaging marker. Moreover, the introduction of new MRI probes and PET tracers should further extend the clinical application of this hybrid methodology, allowing a better understanding of cancer biology.
Methodological considerations
Technical and methodological aspects of PET/MRI are addressed more extensively in another article, in this issue [93]. Certainly, to fully explore the clinical applications of this imaging modality, several methodological problems should be addressed. Briefly, the quality of PET/MR imaging, particularly of PET imaging, should be comparable to that obtained with PET/CT, including anatomical localization and semi-quantitative data. In addition, in oncology, optimization of the scan protocol, workflow and image analysis is more complex for integrated PET/MRI than for clinical PET/CT.
In fused PET and MRI imaging, the results of Dixon-based MR imaging, a water/fat separation technique used for MR-based attenuation correction for PET/MRI, were evaluated for anatomical correlation of PET-positive lesions on a 3 T clinical scanner compared to low-dose CT [94]. No significant difference was found in anatomical localization for all PET-positive lesions in low-dose CT compared to Dixon-based MR [94]. Moreover, SUV obtained from PET/MRI showed a high concordance with SUV from PET/CT, 6.31 ± 4.52 vs 6.36 ± 4.47 [94]. Fully integrated PET/MRI showed more than encouraging results in patients with oncologic diagnoses with excellent correspondence with PET/CT in terms of image quality, contrast and alignment [95]. No significant difference was found in detection rate both on a lesion and on a patient basis, and quantitative evaluation showed high correlation between SUVs obtained by PET/MRI and PET/CT both in lesions and in background tissue [93]. A high concordance (ranging from 88 to 99 %) between PET/MRI and PET/CT on both a patient and a lesion basis was observed by other authors [96, 97].
Thus, integrated clinical whole-body PET/MRI is feasible, producing images of high quality. Despite different attenuation approaches, qualitative and quantitative image analysis showed excellent correlation with PET/CT.
Quantitative PET is playing an increasing role in oncology, and different approaches have been used to quantify tracer uptake by PET/CT: from full kinetic analysis to SUV (SUVmean, SUVmax, SUVpeak), metabolic tumor volume and total lesion glycolysis. To date few papers have directly compared semi-quantitative SUV measurements determined using PET/MRI versus PET/CT [95, 96]. Both SUVmax and SUVmean determined by PET/MRI were significantly lower than the corresponding values obtained by PET/CT [95, 96]. This difference was observed in lesions as well as in background, and in specific organs (i.e., liver, lung, bone, spleen, and muscle), and ranged from −11 % in lesions to −33 % in bone and −56 % in liver [95, 96]. Differences in time interval between tracer administration and scan acquisition cannot explain the lower SUV values observed with PET/MRI. Actually, the PET/MRI scans were started later than (56–88 min after) the PET/CT imaging [95, 96]. The fact that FDG uptake expressed as SUV is well known to increase over time in malignant lesions means that, the finding of significantly lower values in lesions detected with PET/MRI is related to other factors. On the other hand, FDG uptake in normal tissue decreases over time, but this decrease is lower than the differences observed in both these studies [95, 96]. Thus, these findings suggest an underestimation of semi-quantitative assessment of tracer uptake in PET/MRI. Many factors influence SUV quantification, and among them attenuation correction may play a major role in PET/MRI. It has recently been reported that increasing the numbers of tissue classes used to produce attenuation maps substantially reduces the relative error in measuring SUV leading to a significant decrease in underestimation of SUV by PET/MRI in both lesions and normal tissue [98, 99]. Further studies are needed to address these issues, and even more importantly, to assess the reproducibility of quantitative data obtained with PET/MRI.
Since functional and molecular data can be obtained using MRI, the design of clinical protocols for whole-body PET/MRI can be more complex than it is for PET/CT. In addition, decisions should be taken on complete or partial body coverage as well as on specific MRI acquisition protocols. Actually, patients can be referred for PET/MRI with prior imaging results, including PET/CT, or without such results. In the first scenario, a partial PET/MRI scan can be applied, with a few bed positions, and a more complete MRI examination, including fMRI. Conversely, when no or few prior imaging results are available, a whole-body PET/MRI is mandatory, followed by specific MRI sequences to address specific questions raised by the whole-body scan. It should be underlined that each choice will have an impact on imaging time, workflow and image analysis. Moreover, while no particular artifacts are to be expected in MRI images, MRI-based attenuation correction would produce specific artifacts on PET images due to metallic implants, truncation, particular tissue effects (i.e., bone and lung), and the misregistration that is occasionally present in integrated PET/MRI. A first paper dealing with workflow and scan protocols in oncology with a specific integrated PET/MRI scanner has been published [100], and similar efforts for other commercial scanners are expected in the near future.
Conclusions
PET/MRI has the potential to repeat the success of PET/CT in clinical oncology, particularly in those indications, such as soft-tissue tumors, where MRI offers advantages over CT. The data obtained from fMRI appears complementary to the information provided by PET, either using FDG or non-FDG tracers. Although the literature on integrated PET/MRI in oncology is limited, the first experiences with this technique as well as data from fused PET and MRI imaging support its diagnostic utility. Moreover, a clear advantage of PET/MRI over PET/CT is the lower exposure of patients to radiation. PET/CT delivers a relatively high-absorbed radiation dose, compared with the dose from a regular chest radiograph. Since MRI does not use any ionizing radiation, it can conceivably be used without restrictions in serial studies, in children, and in all these situations in which radiation exposure could be a concern. Thus, it is conceivable that PET/MRI could replace PET/CT in all these settings.
However, at a time when the value of PET/CT is still questioned by many health administrators on the basis of cost concerns, the development and the subsequent clinical introduction of PET/MRI, a more expensive and more complex imaging modality, will pose new and major challenges for physicians, industries and administrators. Scientific literature, able to give answers to specific questions in particular clinical settings, is awaited before routine clinical oncology applications of PET/MRI can be proposed. Whether PET/MRI will replace PET/CT or be a complementary methodology depends on what value is added by PET/MRI to cancer imaging, showing the modality to constitute true progress. Moreover, cost-efficiency issues will play a major role in the decision-making process. On the basis of current knowledge, it can be anticipated that hybrid PET/MRI will probably not replace PET/CT in all cases, but rather be used as an integration in some applications and as a stand-alone hybrid technology in other indications and in children.
References
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R et al (2000) A combined PET/CT scanner for clinical oncology. J Nucl Med 41:1369–1379
Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK (2002) PET diagnostic accuracy: improvement within-line PET–CT system: initial results. Radiology 225:575–581
Mittra E, Quon A (2009) PET/CT: the current technology and applications. Radiol Clin North Am 47:147–160
Patel CN, Goldstone AR, Chowdhury FU, Scarsbrook AF (2010) FDG PET/CT in oncology: ‘‘raising the bar’’. Clin Radiol 65:522–535
Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME (2001) A tabulated summary of the FDG PET literature. J Nucl Med 42(5 Suppl):1S–93S
Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, Bockisch A, Debatin JF, Freudenberg LS (2004) Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-d-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol 22(21):4357–4368
Von Schulthess GK, Steinert HC, Hany TF (2006) Integrated PET/CT: current applications and future directions. Radiology 238:405–422
Blodgett TM, Meltzer CC, Townsend DW (2007) PET/CT: form and function. Radiology 242:360–385
Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G (2009) PET/CT for staging and follow-up of patients with malignancy. Eur J Radiol 70:382–392
Bettinardi V, Picchio M, Di Muzio N, Gianolli L, Messa C, Gilardi MC (2010) PET/CT for radiotherapy: image acquisition and data processing. Q J Nucl Med Mol Imaging 54(5):455–475
Seemann MD (2005) Whole-body PET/MRI: the future in oncological imaging. Technol Cancer Res Treat 4:577–582
Zaidi H, Mawlawi O, Orton CG (2007) Simultaneous PET/MR will replace PET/CT as the molecular multimodality imaging platform of choice. Med Phys 34:1525–1528
von Schulthess GK, Schlemmer HPW (2009) A look ahead: PET/MR versus PET/CT. Eur J Nucl Med Mol Imaging 36(Suppl. 1):S3–S9
Shao Y, Cherry SR, Farahani K, Meadors K, Siegel S, Silverman RW, Marsden PK (1997) Simultaneous PET and MR imaging. Phys Med Biol 42:1965–1970
Delso G, Ziegler S (2009) PET/MRI system design. Eur J Nucl Med Mol Imaging 36(suppl 1):S86–S92
Herzog H (2012) PET/MRI: challenges, solutions and perspectives. Z Med Phys 22:281–298. doi:10.1016/j.zemedi.2012.07.003
Yoon HS, Ko GB, Kwon SI, Lee CM, Ito M, Chan Song I, Lee DS, Hong SJ, Lee JS (2012) Initial results of simultaneous PET/MRI experiments with an MRI-compatible silicon photomultiplier PET scanner. J Nucl Med 53:608–614
Zaid H, Del Guerra A (2011) An outlook on future design of hybrid PET/MRI systems. Med Phys 38:5667–5689
Kops ER, Herzog H (2007) Alternative methods for attenuation correction for PET images in MR-PET scanners. IEEE Nucl Sci Symp Conf Rec 6:4327–4330
Zaidi H, Montandon M, Slosman DO (2003) Magnetic resonance imaging-guided attenuation and scatter corrections in three-dimensional brain positron emission tomography. Med Phys 30:937–948
Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, Brady M, Schölkopf B, Pichler BJ (2008) MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med 49:1875–1883
Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke S, Beyer T, Pichler BJ, Bernhard Scholkopf B (2011) MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med 52:1392–1399
Martinez-Möller A, Nekolla SG (2012) Attenuation correction for PET/MR: problems, novel approaches and practical solutions. Z Med Phys 22:299–310. doi:10.1016/j.zemedi.2012.08.003
Schlemmer HP, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, Jattke K, Townsend D, Nahmias C, Jacob PK, Heiss WD, Claussen CD (2008) Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology 248:1028–1035
Pauls S, Buck AK, Hohl K, Halter G, Hetzel M, Blumstein NM, Mottaghy FM, Glatting G, Krüger S, Sunder-Plassmann L, Möller P, Hombach V, Brambs HJ, Reske SN (2007) Improved non-invasive T-staging in non-small cell lung cancer by integrated 18F-FDG PET/CT. Nuklearmedizin 46(1):9–14
Babin E, Desmonts C, Hamon M, Bénateau H, Hitier M (2008) PET/CT for assessing mandibular invasion by intraoral squamous cell carcinomas. Clin Otolaryngol 33(1):47–51
Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Börsch G, Dahmen G, Barkhausen J, Bockisch A, Antoch G (2006) Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA 296(21):2590–2600
Mainenti PP, Iodice D, Segreto S, Storto G, Magliulo M, De Palma GD, Salvatore M, Pace L (2011) Colorectal cancer and 18FDG-PET/CT: what about adding the T to the N parameter in loco-regional staging? World J Gastroenterol 17(11):1427–1433
Boss A, Stegger L, Bisdas S, Kolb A, Schwenzer N, Pfister M, Claussen CD, Pichler BJ, Pfannenberg C (2011) Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol 21:1439–1446
Nakamoto Y, Tamai K, Saga T, Higashi T, Hara T, Suga T, Koyama T, Togashi K (2009) Clinical value of image fusion from MR and PET in patients with head and neck cancer. Mol Imaging Biol 11:46–53
Kuhl C (2007) The current status of breast MR imaging. Part 1. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 244:356–378
Imbriaco M, Caprio MG, Limite G, Pace L, De Falco T, Capuano E, Salvatore M (2008) Dual-time-point 18F-FDG PET/CT versus dynamic breast MRI of suspicious breast lesions. Am J Roentgenol 191(5):1323–1330
Moy L, Noz ME, Maguire GQ Jr, Melsaether A, Deans AE, Murphy-Walcott AD, Ponzo F (2010) Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J 16:369–376
Hahn S, Heusner T, Kümmel S, Köninger A, Nagarajah J, Müller S, Boy C, Forsting M, Bockisch A, Antoch G, Stahl A (2011) Comparison of FDG-PET/CT and bone scintigraphy for detection of bone metastases in breast cancer. Acta Radiol 52:1009–1014
Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH (2008) A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 49:1912–1921
Kam MH, Wong DC, Siu S, Stevenson AR, Lai J, Phillips GE (2010) Comparison of magnetic resonance imaging-fluorodeoxy-glucose positron emission tomography fusion with pathological staging in rectal cancer. Br J Surg 97:266–268
Hogendoorn PC; ESMO/EUROBONET Working Group, Athanasou N, Bielack S, De Alava E, Dei Tos AP, Ferrari S, Gelderblom H, Grimer R, Hall KS, Hassan B, Hogendoorn PC, Jurgens H, Paulussen M, Rozeman L, Taminiau AH, Whelan J, Vanel D (2010) Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(suppl 5):204–213
Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Kim EE (2007) Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 245:839–847
Sinha S, Peach AH (2010) Diagnosis and management of soft tissue sarcoma. BMJ 341:c7170
Tateishi U, Hosono A, Makimoto A, Nakamoto Y, Kaneta T, Fukuda H, Murakami K, Terauchi T, Suga T, Inoue T, Kim EE (2009) Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann Nucl Med 23:155–161
Thorwarth D, Leibfarth S, Mönnich D (2013) Potential role of PET/MRI in radiotherapy treatment planning. Clin Transl Imaging. doi:10.1007/s40336-013-0006-2
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V; International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD; Imaging Subcommittee of International Harmonization Project in Lymphoma (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25:571–578
Wu LM, Chen FY, Jiang XX, Gu HY, Yin Y, Xu JR (2012) 18F-FDG PET, combined FDG-PET/CT and MRI for evaluation of bone marrow infiltration in staging of lymphoma: a systematic review and meta-analysis. Eur J Radiol 81:303–311
Punwani S, Taylor SA, Bainbridge A, Prakash V, Bandula S, De Vita E, Olsen OE, Hain SF, Stevens N, Daw S, Shankar A, Bomanji JB, Humphries PD (2010) Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology 255:182–190
Lin C, Luciani A, Itti E, El-Gnaoui T, Vignaud A, Beaussart P, Lin SJ, Belhadj K, Brugières P, Evangelista E, Haioun C, Meignan M, Rahmouni A (2010) Whole-body diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient mapping for staging patients with diffuse large B-cell lymphoma. Eur Radiol 20:2027–2038
van Ufford HM, Kwee TC, Beek FJ, van Leeuwen MS, Takahara T, Fijnheer R, Nievelstein RA, de Klerk JM (2011) Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18F-FDG PET/CT. AJR 196:662–669
Pfluger T, Melzer HI, Mueller WP, Coppenrath E, Bartenstein P, Albert MH, Schmid I (2012) Diagnostic value of combined 18F-FDG PET/MRI for staging and restaging in paediatric oncology. Eur J Nucl Med Mol Imaging 39:1745–1755
Collins CD (2007) PET/CT in oncology: for which tumours is it the reference standard? Cancer Imaging 7(Spec No A):S77–S87
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, Dahmen G (2003) Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 290:3199–3206
Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E (2012) Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 19:2212–2223
Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ (2011) Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDGPET/CT. J Comput Assist Tomogr 35:531–534
Volker T, Denecke T, Steffen I et al (2007) Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol 25:5435–5441
Dummer R, Hauschild A, Guggenheim M, Jost L, Pentheroudakis G; ESMO Guidelines Working Group (2010) Melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(suppl 5):v194–v197
Schmidt GP, Baur-Melnyk A, Haug A, Heinemann V, Bauerfeind I, Reiser MF, Schoenberg SO (2008) Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET–CT. Eur J Radiol 65(1):47–58
Pfannenberg C, Aschoff P, Schanz S, Eschmann SM, Plathow C, Eigentler TK, Garbe C, Brechtel K, Vonthein R, Bares R, Claussen CD, Schlemmer HP (2007) Prospective comparison of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced malignant melanoma. Eur J Cancer 43:557–564
Schmidt GP, Schoenberg SO, Schmid R, Stahl R, Tiling R, Becker CR, Reiser MF, Baur-Melnyk A (2007) Screening for bone metastases: whole-body MRI using a 32-channel system versus dual-modality PET–CT. Eur Radiol 17:939–949
Schmidt GP, Baur-Melnyk A, Herzog P, Schmid R, Tiling R, Schmidt M, Reiser MF, Schoenberg SO (2005) High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography–computed tomography: experience on a 32-channel system. Invest Radiol 40:743–753
Liu J, Yang X, Li F, Wang X, Jiang X (2011) Preliminary study of whole-body diffusion-weighted imaging in detecting pulmonary metastatic lesions from clear cell renal cell carcinoma: comparison with CT. Acta Radiol 52:954–963
Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE (2011) Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol 46:548–555
Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B, Weishaupt D (2010) Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med 51:692–699
Yang HL, Liu T, Wang XM, Xu Y, Deng SM (2011) Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 21:2604–2617
Liu T, Cheng T, Xu W, Yan WL, Liu J, Yang HL (2011) A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol 40:523–531
Qu X, Huang X, Yan W, Wu L, Dai K (2012) A meta-analysis of 18FDG-PET–CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur J Radiol 81:1007–1015
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Weber WA (2009) Assessing tumor response to therapy. J Nucl Med 50:1S–10S
Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E et al (2012) Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int J Radiat Oncol Biol Phys 82:863–870
Dong S, Ye XD, Yuan Z, Xu LC, Xiao XS (2012) Relationship of apparent diffusion coefficient to survival for patients with unresectable primary hepatocellular carcinoma after chemoembolization. Eur J Radiol 81:472–477
Vandecaveye V, Dirix P, De Keyzer F, Op de Beeck K, Vander Poorten V, Hauben E, Lambrecht M, Nuyts S, Hermans R (2012) Diffusion-weighted magnetic resonance imaging early after chemoradiotherapy to monitor treatment response in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 82:1098–1107
Nilsen L, Fangberget A, Geier O, Olsen DR, Seierstad T (2010) Diffusion-weighted magnetic resonance imaging for pretreatment prediction and monitoring of treatment response of patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Acta Oncol 49:354–360
Chen JH, Bahri S, Mehta RS, Kuzucan A, Yu HJ, Carpenter PM, Feig SA, Lin M, Hsiang DJ, Lane KT, Butler JA, Nalcioglu O, Su MY (2011) Breast cancer: evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology 261:735–743
Ueda S, Tsuda H, Saeki T, Osaki A, Shigekawa T, Ishida J, Tamura K, Abe Y, Omata J, Moriya T, Fukatsu K, Yamamoto J (2010) Early reduction in standardized uptake value after one cycle of neoadjuvant chemotherapy measured by sequential FDG PET/CT is an independent predictor of pathological response of primary breast cancer. Breast J 16:660–662
Bolog N, Pfammatter T, Mullhaupt B, Andreisek G, Weishaupt D (2008) Double-contrast magnetic resonance imaging of hepatocellular carcinoma after transarterial chemoembolization. Abdom Imaging 33:313–323
Veit P, Antoch G, Stergar H, Bockisch A, Forsting M, Kuehl H (2006) Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: initial results. Eur Radiol 16:80–87
Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H (2011) Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 260:771–780
de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ (2009) Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med 50(Suppl 1):43S–54S
Pregno P, Chiappella A, Bellò M, Botto B, Ferrero S, Franceschetti S, Giunta F, Ladetto M, Limerutti G, Menga M, Nicolosi M, Priolo G, Puccini B, Rigacci L, Salvi F, Vaggelli L, Passera R, Bisi G, Vitolo U (2012) Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 119:2066–2073
Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL (2011) 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med 52:386–392
Lin C, Itti E, Luciani A, Zegai B, Lin SJ, Kuhnowski F, Pigneur F, Gaillard I, Paone G, Meignan M, Haioun C, Rahmouni A (2011) Whole-body diffusion-weighted imaging with apparent diffusion coefficient mapping for treatment response assessment in patients with diffuse large B-cell lymphoma: pilot study. Invest Radiol 46:341–349
Inaoka T, Takahashi K, Mineta M, Yamada T, Shuke N, Okizaki A, Nagasawa K, Sugimori H, Aburano T (2007) Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 243:869–876
Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, Pfannenberg C, Pichler BJ, Reimold M, Stegger L (2010) Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med 51:1198–1205
Ledezma CJ, Chen W, Sai V, Freitas B, Cloughesy T, Czernin J, Pope W (2009) 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience. Eur J Radiol 71:242–248
Nagarajah J, Jentzen W, Hartung V, Rosenbaum-Krumme S, Mikat C, Heusner TA, Antoch G, Bockisch A, Stahl A (2011) Diagnosis and dosimetry in differentiated thyroid carcinoma using 124I PET: comparison of PET/MRI vs PET/CT of the neck. Eur J Nucl Med Mol Imaging 38:1862–1868
Verma S, Rajesh A (2011) A clinically relevant approach to imaging prostate cancer: review. Am J Roentgenol 196(3 Suppl):S1–S10
Jadvar H (2011) Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med 52:81–89
Park H, Wood D, Hussain H, Meyer CR, Shah RB, Johnson TD, Chenevert T, Piert M (2012) Introducing parametric fusion PET/MRI of primary prostate cancer. J Nucl Med 53:546–551
Jambor I, Borra R, Kemppainen J, Lepomäki V, Parkkola R, Dean K, Alanen K, Arponen R, Nurmi M, Aronen HJ, Heikki Minn H (2012) Improved detection of localized prostate cancer using co-registered MRI and 11C-acetate PET/CT. Eur J Radiol 81:2966–2972. doi:10.1016/j.ejrad.2011.12.043
Nanni C, Fantini L, Nicolini S, Fanti S (2010) Non FDG PET. Clin Radiol 65:536–548
Kumar R, Dhnapathi H, Basu S, Rubello D, Fanti S, Alavi A (2008) Oncologic PET tracers beyond (18F)FDG and the novel quantitative approaches in PET imaging. Q J Nucl Med Mol Imaging 52:50–65
Sauter AW, Wehrl HF, Kolb A, Judenhofer MS, Pichler BJ (2010) Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med 16(11):508–515
Ziegler SI, Delso G (2013) Technical and methodological aspects of PET/MRI. Clin Transl Imaging. doi:10.1007/s40336-013-0011-5
Eiber M, Martinez-Möller A, Souvatzoglou M, Holzapfel K, Pickhard A, Löffelbein D, Santi I, Rummeny EJ, Ziegler S, Schwaiger M, Nekolla SG, Beer AJ (2011) Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging 38:1691–1701
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Moller A, Nekolla SG, Ziegler S, Ganter C, Rummeny EJ, Schwaiger M (2012) First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med 53(6):845–855. doi:10.2967/jnumed.111.098608
Wiesmüller M, Quick HH, Navalpakkam B, Lell MM, Uder M, Ritt P, Schmidt D, Beck M, Kuwert T, von Gall CC (2013) Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging 40:12–21
Platzek I, Beuthien-Baumann B, Schneider M, Gudziol V, Langner J, Schramm G, Laniado M, Kotzerke J, van den Hoff J (2013) PET/MRI in head and neck cancer: initial experience. Eur J Nucl Med Mol Imaging 40:6–11
Akbarzadeh A, Ay MR, Ahmadian A, Riahi Alam N, Zaidi H (2013) MRI-guided attenuation correction in whole-body PET/MR: assessment of the effect of bone attenuation. Ann Nucl Med 27(2):152–162
Kim JH, Lee JS, Song IC, Lee DS (2012) Comparison of segmentation-based attenuation correction methods for PET/MRI: evaluation of bone and liver standardized uptake value with oncologic PET/CT data. J Nucl Med 53:1878–1882
Martinez-Moller A, Eiber M, Nekolla SG, Souvatzoglou M, Drzezga A, Ziegler S, Rummeny EJ, Schwaiger M, Beer AJ (2012) Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology. J Nucl Med 53:1415–1426
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pace, L., Nicolai, E., Aiello, M. et al. Whole-body PET/MRI in oncology: current status and clinical applications. Clin Transl Imaging 1, 31–44 (2013). https://doi.org/10.1007/s40336-013-0012-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-013-0012-4