Abstract
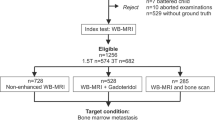
Numerous imaging modalities are available for the diagnosis of bone metastases (BM) in patients with cancer. This paper reviews published studies comparing whole-body (wb) MRI to BS, PET or PET-CT to provide up-to-date evaluation of the diagnostic accuracy of wbMRI to detect BM. This review is based on a systematic search of the literature of studies evaluating the diagnostic performance of wbMRI for BM detection. Evidence synthesis was achieved via a classification tree, which provides an overview of the performance of the different imaging techniques and of the context in which these are assessed, and an evidence table to seek confirmation of the diagnostic value of the imaging techniques. Eligible studies (n = 23/301) included patients with cancer who underwent wbMRI for suspicion of BM or for systematic cancer staging, compared with at least one other imaging tool. The evidence supporting the superiority of wbMRI over BS for BM screening is strong. wbMRI with diffusion-weighted imaging (DWI) sequence has higher sensitivity than wbMRI alone. The respective accuracies of wbMRI and FDG PET-CT differ depending both on the type of analysis (lesion, region or patient-based) and primary cancer. wbMRI and PET-CT emerge as the techniques of choice for the diagnosis of BM. The inclusion of DWI in wbMRI protocols is of value to optimize the sensitivity but does not add in specificity. This indicates the importance of the anatomical sequences in those protocols. Further comparisons between wbMRI and DWI alone, 18F-NaF, 11C-Choline, PSMA PET-CT and PET-MRI systems remain necessary.



Similar content being viewed by others
Abbreviations
- Se:
-
Sensitivity
- Sp:
-
Specificity
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- wbMRI:
-
Whole-body magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- STIR:
-
Short tau inversion recovery
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- BS:
-
Bone scintigraphy
- BM:
-
Bone metastases
- SD:
-
Statistical difference between capability (Se/Sp) values assessed at a standard p value <0.05
- bSD:
-
Statistical difference between capability values assessed at a p value compliant with Bonferroni’s correction
- nsSD:
-
Statistical difference between capability values superior or equal to 5 % assessed qualitatively or reported to be statistically non-significant (p > 0.05)
- Eq:
-
Statistical difference between capability values inferior to 5 % assessed qualitatively or reported to be statistically non-significant (p > 0.05)
References
Padhani AHJ (1998) Imaging in oncology/bone metastases. Isis Medical Media, Oxford, pp 765–787
Bronner F (2009) Bone and cancer. Springer, London
Schirrmeister H, Guhlmann A, Kotzerke J et al (1999) Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol 17:2381–2389
Even-Sapir E, Metser U, Mishani E et al (2006) The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 47:287–297
Savelli G, Maffioli L, Maccauro M et al (2001) Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q J Nucl Med 45:27–37
Kim EE (2013) Clinical PET and PET/CT principles and applications. Springer, New York
Walker R, Kessar P, Blanchard R et al (2000) Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging 11:343–350
Lauenstein TC, Freudenberg LS, Goehde SC et al (2002) Whole-body MRI using a rolling table platform for the detection of bone metastases. Eur Radiol 12:2091–2099
Lecouvet FE, Geukens D, Stainier A et al (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25:3281–3287
Freedman GM, Negendank WG, Hudes GR et al (1999) Preliminary results of a bone marrow magnetic resonance imaging protocol for patients with high-risk prostate cancer. Urology 54:118–123
Ghanem N, Altehoefer C, Hogerle S et al (2002) Comparative diagnostic value and therapeutic relevance of magnetic resonance imaging and bone marrow scintigraphy in patients with metastatic solid tumors of the axial skeleton. Eur J Radiol 43:256–261
Engelhard K, Hollenbach HP, Wohlfart K et al (2004) Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol 14:99–105
Nakanishi K, Kobayashi M, Nakaguchi K et al (2007) Whole-body MRI for detecting metastatic bone tumor: diagnostic value of diffusion-weighted images. Magn Reson Med Sci 6:147–155
Kwee TC, Takahara T, Ochiai R et al (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Pearce T, Philip S, Brown J et al (2012) Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br J Radiol 85:1102–1106
Thoeny HC, De Keyzer F (2007) Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol 17:1385–1393
Muller MF, Prasad P, Siewert B et al (1994) Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology 190:475–478
Muller MF, Prasad PV, Bimmler D et al (1994) Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology 193:711–715
Hovels AM, Heesakkers RA, Adang EM et al (2008) The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 63:387–395
Lecouvet FE, El Mouedden J, Collette L et al (2012) Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 62:68–75
Pasoglou V, Larbi A, Collette L et al (2014) One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? Prostate 74:469–477
Costelloe CM, Kundra V, Ma J et al (2012) Fast Dixon whole-body MRI for detecting distant cancer metastasis: a preliminary clinical study. J Magn Reson Imaging 35:399–408
Pasoglou V, Michoux N, Peeters F et al. (2014) Whole-body 3D T1-weighted MR imaging in patients with prostate cancer: feasibility and evaluation in screening for metastatic disease. Radiology 275(1):155–166
Takahara TIY, Yamashita T, Yasuda S, Nasu S, Van Cauteren M (2004) Diffusion weighted whole body imaging with backgraound body signal suppression (DWIBS): technical impovement using free breathing STIR and high resolution 3D display. Radiat Med 22:275–282
Berlin JA, Rennie D (1999) Measuring the quality of trials: the quality of quality scales. JAMA 282:1083–1085
Greenland S (1994) Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 140:290–296
Bossuyt PM, Reitsma JB (2003) Standards for reporting of diagnostic A: the STARD initiative. Lancet 361:71
Whiting P, Rutjes AW, Reitsma JB et al (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Antoch G, Vogt FM, Freudenberg LS et al (2003) Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 290:3199–3206
Balliu E, Boada M, Pelaez I et al (2010) Comparative study of whole-body MRI and bone scintigraphy for the detection of bone metastases. Clin Radiol 65:989–996
Daldrup-Link HE, Franzius C, Link TM et al (2001) Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol 177:229–236
Eustace S, Tello R, DeCarvalho V et al (1997) A comparison of whole-body turboSTIR MR imaging and planar 99mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol 169:1655–1661
Goo HW, Choi SH, Ghim T et al (2005) Whole-body MRI of paediatric malignant tumours: comparison with conventional oncological imaging methods. Pediatr Radiol 35:766–773
Gutzeit A, Doert A, Froehlich JM et al (2010) Comparison of diffusion-weighted whole body MRI and skeletal scintigraphy for the detection of bone metastases in patients with prostate or breast carcinoma. Skeletal Radiol 39:333–343
Heusner TA, Kuemmel S, Koeninger A et al (2010) Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging 37:1077–1086
Heusner T, Golitz P, Hamami M et al (2011) “One-stop-shop” staging: should we prefer FDG-PET/CT or MRI for the detection of bone metastases? Eur J Radiol 78:430–435
Jouvet JC, Thomas L, Thomson V et al (2013) Whole-body MRI with diffusion-weighted sequences compared with 18 FDG PET-CT: CT and superficial lymph node ultrasonography in the staging of advanced cutaneous melanoma: a prospective study. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12078
Kembhavi SA, Rangarajan V, Shah S et al (2014) Prospective observational study on diagnostic accuracy of whole-body MRI in solid small round cell tumours. Clin Radiol 69:900–908
Kumar J, Seith A, Kumar A et al (2008) Whole-body MR imaging with the use of parallel imaging for detection of skeletal metastases in pediatric patients with small-cell neoplasms: comparison with skeletal scintigraphy and FDG PET/CT. Pediatr Radiol 38:953–962
Laurent V, Trausch G, Bruot O et al (2010) Comparative study of two whole-body imaging techniques in the case of melanoma metastases: advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET-CT. Eur J Radiol 75:376–383
Ohno Y, Koyama H, Nogami M et al (2007) Whole-body MR imaging vs. FDG-PET: comparison of accuracy of M-stage diagnosis for lung cancer patients. J Magn Reson Imaging 26:498–509
Pfannenberg C, Aschoff P, Schanz S et al (2007) Prospective comparison of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced malignant melanoma. Eur J Cancer 43:557–564
Sakurai Y, Kawai H, Iwano S et al (2013) Supplemental value of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) technique to whole-body magnetic resonance imaging in detection of bone metastases from thyroid cancer. J Med Imaging Radiat Oncol 57:297–305
Schmidt GP, Schoenberg SO, Schmid R et al (2007) Screening for bone metastases: whole-body MRI using a 32-channel system versus dual-modality PET-CT. Eur Radiol 17:939–949
Schraml C, Schwenzer NF, Sperling O et al (2013) Staging of neuroendocrine tumours: comparison of [(6)(8)Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imaging 13:63–72
Sohaib SA, Cook G, Allen SD et al (2009) Comparison of whole-body MRI and bone scintigraphy in the detection of bone metastases in renal cancer. Br J Radiol 82:632–639
Takenaka D, Ohno Y, Matsumoto K et al (2009) Detection of bone metastases in non-small cell lung cancer patients: comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J Magn Reson Imaging 30:298–308
Venkitaraman R, Cook GJ, Dearnaley DP et al (2009) Whole-body magnetic resonance imaging in the detection of skeletal metastases in patients with prostate cancer. J Med Imaging Radiat Oncol 53:241–247
Leboulleux S, Dromain C, Vataire AL et al (2008) Prediction and diagnosis of bone metastases in well-differentiated gastro-entero-pancreatic endocrine cancer: a prospective comparison of whole body magnetic resonance imaging and somatostatin receptor scintigraphy. J Clin Endocrinol Metab 93:3021–3028
Heusner TA, Antoch G, Wittkowski-Sterczewski A et al (2011) Transarterial hepatic chemoperfusion of uveal melanoma metastases: survival and response to treatment. RoFo 183:1151–1160
Schmidt GP, Baur-Melnyk A, Haug A et al (2009) Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol 19:1366–1378
Roberts CC, Daffner RH, Weissman BN et al (2010) ACR appropriateness criteria on metastatic bone disease. J Am Coll Radiol 7:400–409
Cardoso F, Senkus-Konefka E, Fallowfield L et al (2010) Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v15–v19
Khatcheressian JL, Wolff AC, Smith TJ et al (2006) American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24:5091–5097
Briganti A, Passoni N, Ferrari M et al (2010) When to perform bone scan in patients with newly diagnosed prostate cancer: external validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur Urol 57:551–558
Horwich A, Parker C, de Reijke T et al (2013) Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol 24(5):1141–1162
Leibovici D, Spiess PE, Agarwal PK et al (2007) Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 109:198–204
Kosuda S, Yoshimura I, Aizawa T et al (2002) Can initial prostate specific antigen determinations eliminate the need for bone scans in patients with newly diagnosed prostate carcinoma? A multicenter retrospective study in Japan. Cancer 94:964–972
Erturan S, Yaman M, Aydin G et al (2005) The role of whole-body bone scanning and clinical factors in detecting bone metastases in patients with non-small cell lung cancer. Chest 127:449–454
Wu LM, Gu HY, Zheng J et al (2011) Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imaging 34:128–135
Yang HL, Liu T, Wang XM et al (2011) Diagnosis of bone metastases: a meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 21:2604–2617
Shen G, Deng H, Hu S et al (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513
Houssami N, Costelloe CM (2012) Imaging bone metastases in breast cancer: evidence on comparative test accuracy. Ann Oncol 23:834–843
Duo J, Han X, Zhang L et al (2013) Comparison of FDG PET/CT and gadolinium-enhanced MRI for the detection of bone metastases in patients with cancer: a meta-analysis. Clin Nucl Med 38:343–348
Khoo MM, Tyler PA, Saifuddin A et al (2011) Diffusion-weighted imaging (DWI) in musculoskeletal MRI: a critical review. Skeletal Radiol 40:665–681
Schiepers C, Nuyts J, Bormans G et al (1997) Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med 38:1970–1976
Even-Sapir E, Metser U, Flusser G et al (2004) Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med 45:272–278
Li W, Ma L, Wang X et al (2014) C-choline PET/CT tumor recurrence detection and survival prediction in post-treatment patients with high-grade gliomas. Tumour Biol 35:12353–12360
Granov A (2013) Positron emission tomography. Springer, Berlin
Ghosh A, Heston WD (2004) Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 91:528–539
Bacich DJ, Pinto JT, Tong WP et al (2001) Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome 12:117–123
Afshar-Oromieh A, Malcher A, Eder M et al (2013) PET imaging with a [68 Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 40:486–495
Luboldt W, Kufer R, Blumstein N et al (2008) Prostate carcinoma: diffusion-weighted imaging as potential alternative to conventional MR and 11C-choline PET/CT for detection of bone metastases. Radiology 249:1017–1025
Lecouvet FE, Vande Berg BC, Malghem J et al (2009) Diffusion-weighted MR imaging: adjunct or alternative to T1-weighted MR imaging for prostate carcinoma bone metastases? Radiology 252:624
Mosavi F, Johansson S, Sandberg DT et al (2012) Whole-body diffusion-weighted MRI compared with (18)F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am J Roentgenol 199:1114–1120
Afshar-Oromieh A, Avtzi E, Giesel FL et al (2014) The diagnostic value of PET/CT imaging with the Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42:197–209
Carrio I (2014) PET/MRI methodology and clinical applications. Springer, Berlin
Peller P (2012) PET-CT and PET-MRI in oncology: a practical guide. Springer, Berlin
ClinicalTrials.gov. In: Health USNIo, ed. (2014)
Sardanelli F, Di Leo G (2009) Biostatistics for radiologists: planning, performing, and writing a radiologic study. Springer, Italy
Walter SD, Macaskill P, Lord SJ et al (2012) Effect of dependent errors in the assessment of diagnostic or screening test accuracy when the reference standard is imperfect. Stat Med 31:1129–1138
Higgins JP, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 172:137–159
Lecouvet FE, Talbot JN, Messiou C et al (2014) Monitoring the response of bone metastases to treatment with magnetic resonance imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer 50:2519–2531
Schmidt GP, Baur-Melnyk A, Herzog P et al (2005) High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography-computed tomography: experience on a 32-channel system. Invest Radiol 40:743–753
Acknowledgments
Supported by Grants from the Belgian non-profit organizations FRS-FNRS Télévie, Fondation Contre le Cancer and Fondation Saint Luc to VP and FEL
Conflict of interest
V. Pasoglou, N. Michoux, B. Tombal, F. Jamar, F.E. Lecouvet declare no conflict of interest.
Compliance with ethics guidelines
Critical review of human study.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Pasoglou and N. Michoux contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pasoglou, V., Michoux, N., Tombal, B. et al. wbMRI to detect bone metastases: critical review on diagnostic accuracy and comparison to other imaging modalities. Clin Transl Imaging 3, 141–157 (2015). https://doi.org/10.1007/s40336-015-0120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-015-0120-4