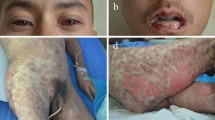
Abstract
Background
Severe skin reactions, mostly following medication use, are rare and can be associated with high mortality. A suitable treatment approach that is able to reduce mortality is needed.
Methods
Recent publications on this topic were reviewed and evaluated.
Results
In the case of the self-limiting diseases acute generalized exanthematous pustulosis (AGEP) and generalized bullous fixed drug eruption (GBFDE), there is no clear indication for systemic immunomodulating treatment, and supportive care remains the gold standard. The situation is less clear in the case of drug reaction with eosinophilia and systemic symptoms (DRESS); nevertheless, primarily in the case of severe organ involvement, systemic glucocorticosteroids are recommended. This is associated with complications and often also with virus reactivation, which may delay healing. The evidence on various immunomodulating therapies in Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) is controversial. Recent publications favor steroid pulse treatment, the tumor necrosis factor (TNF)-α inhibitor etanercept, as well as the calcineurin inhibitor cyclosporine A, with the latter representing the most promising approach.
Conclusion
The rarity and unpredictability of the reactions make a randomized double-blind therapeutic trial extremely difficult. Using meta-analyses, it is possible to trace a trend in the use of systemic treatment options. Supportive care remains the most important treatment strategy for all clinical entities.






Similar content being viewed by others
Abbreviations
- AGEP:
-
Acute generalized exanthematous pustulosis
- ANA:
-
Antinuclear antibodies
- BSA:
-
Body surface area
- BW:
-
Body weight
- CI:
-
Confidence interval
- CMV:
-
Cytomegalovirus
- DIHS:
-
Drug-induced hypersensitivity syndrome
- DRESS:
-
Drug reaction with eosinophilia and systemic symptoms
- EBV:
-
Epstein-Barr virus
- EM:
-
Erythema multiforme
- EMM:
-
Erythema multiforme majus
- GBFDE:
-
Generalized bullous fixed drug eruption
- HHV‑6:
-
Human herpesvirus 6
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- IVIG:
-
Intravenous immunoglobulins
- NSAID:
-
Non-steroidal anti-inflammatory drugs
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- SCORTEN:
-
Severity-of-illness score for TEN
- SJS:
-
Stevens-Johnson syndrome
- SSSS:
-
Staphylococcal scalded skin syndrome
- Teffs:
-
Effector T cells
- TEN:
-
Toxic epidermal necrolysis
- Th:
-
T helper cells
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
References
Mockenhaupt M, Roujeau JC. Epidermal Necrolysis (Stevens-Johnson syndrome and toxic epidermal Necrolysis). In: Kang S, Amagai M, Bruckner A, Enk AH, Margolis DJ, McMichael AJ, Orringer JS, editors. Fitzpatrick’s dermatology. 9th ed. New York: McGraw-Hill; 2019. pp. 33–48.
Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49:769–73.
Sidoroff A, Halevy S, Bouwes Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113–9.
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–11.
Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223–32.
Burrows NP, Russell Jones RR. Pustular drug eruptions: a histopathological spectrum. Histopathology. 1993;22:569–73.
Halevy S, Kardaun SH, Davidovici B, Wechsler J, EuroSCAR, RegiSCAR study group. The spectrum of histopathological features in acute generalized exanthematous pustulosis: a study of 102 cases. Br J Dermatol. 2010;163:1245–52.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250–7.
Kano Y, Ishida T, Hirahara K, Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010;94:743–59.
Chi MH, Hui RCY, Yang CH, Lin JY, Lin YT, Ho HC, et al. Histopathological analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br J Dermatol. 2014;170:866–73.
Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, Wechsler J, de Feraudy S, Duong TA, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50–8.
Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083–4.
Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625–45.
Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau J‑C, et al. Severe cutaneous adverse reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138:1019–24.
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6.
Rzany B, Hering O, Mockenhaupt M, Schröder W, Goerttler E, Ring J, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme majus, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 1996;135:6–11.
Ziemer M, Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. Skin Biopsy Perspect. 2011; https://doi.org/10.5772/22335.
Cho YT, Lin JW, Chen YC, Chang CY, Hsiao CH, Chung WH, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539–48.
Paulmann M, Mockenhaupt M. Unintended rechallenge: Generalized bullous fixed drug eruption in two elderly women. Hautarzt. 2017;68:59–63.
Lipowicz S, Sekula P, Ingen-Housz-Oro S, Liss Y, Sassolas B, Dunant A, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726–32.
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JNB, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989–96.
Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363–5.
Davidovici B, Dodiuk-Gad R, Rozenman D, Halevy S, Israeli RegiSCAR Network. Profile of acute generalized exanthematous pustulosis in Israel during 2002–2005: results of the RegiSCAR Study. Isr Med Assoc J. 2008;10:410–2.
Alniemi DT, Wetter DA, Bridges AG, El-Azhary RA, Davis MDP, Camilleri MJ, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996–2013. Int J Dermatol. 2017;56:405–14.
Brockow K, Ardern-Jones MR, Mockenhaupt M, Aberer W, Barbaud A, Caubet JC, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019;74:14–27.
Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333–8.
Davidovici BB, Pavel D, Cagnano E, Rozenman D, Halevy S, EuroSCAR, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525–9.
Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583–4.
Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8.
Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–9.
Paulmann M, Mockenhaupt M. Fever in Stevens-johnson syndrome and toxic epidermal Necrolysis in pediatric cases: laboratory work-up and antibiotic therapy. Pediatr Infect Dis J. 2017;36:513–5.
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44.
Quirke KP, Beck A, Gamelli RL, Mosier MJ. A 15-year review of pediatric toxic epidermal necrolysis. J Burn Care Res. 2015;36:130–6.
Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatr Electron Pages. 2009;123:e297–e304.
Kauppinen K, Stubb S. Fixed eruptions: causative drugs and challenge tests. Br J Dermatol. 1985;112:575–8.
de Argila D, Angeles Gonzalo M, Rovira I. Carbamazepine-induced fixed drug eruption. Allergy. 1997;52:1039.
Baird BJ, De Villez RL. Widespread bullous fixed drug eruption mimicking toxic epidermal necrolysis. Int J Dermatol. 1988;27:170–4.
Dharamsi FM, Michener MD, Dharamsi JW. Bullous fixed drug eruption masquerading as recurrent Stevens-Johnson syndrome. J Emerg Med. 2015;48:551–4.
Schmid S, Kuechler PC, Britschgi M, Steiner UC, Yawalkar N, Limat A, et al. Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation. Am J Pathol. 2002;161:2079–86.
Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, Barker JN, Capon F, Creamer D, et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol. 2013;133:1904–7.
Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized Exanthematous Pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;27(17):8–E1214.
Musette P, Janela B. New insights into drug reaction with Eosinophilia and systemic symptoms Pathophysiology. Front Med. 2017;4:179.
Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DIHS) / drug reactionwith eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68:301–8.
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9.
White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6:38–69.
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–50.
Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal Necrolysis. J Invest Dermatol. 2017;137:1065–73.
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.
Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, et al. A marker for Stevens-Johnson syndrome …: ethnicity matters. Pharmacogenomics J. 2006;6:265–8.
Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA‑B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmaco Genom. 2008;18:99–107.
Roujeau JC, Dunant A, Mockenhaupt M. Epidermal necrolysis, ocular complications, and “cold medicines”. J Allergy Clin Immunol Pract. 2018;6:703–4.
Genin E, Schumacher M, Roujeau J, Naldi L, Liß Y, Kazma R, et al. Genome-wide association study of Stevens-Johnson syndrome and toxic epidermal Necrolysis in europe. Orphan J Rare Dis. 2011;6:52.
Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology. 2014;83(22):2077–84.
Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol. 2002;2:317–23.
Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by selfinflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201–8.
Ghislain P‑D, Roujeau J‑C. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatol Online J. 2002;8:5.
Uhara H, Saiki M, Kawachi S, Ashida A, Oguchi S, Okuyama R. Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J Eur Acad Dermatol Venereol. 2013;27:722–6.
Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–8.
Cho YT, Chu CY. Treatments for severe cutaneous adverse reactions. J Immunol Res. 2017. https://doi.org/10.1155/2017/1503709.
Funck-Brentano E, Duong T‑A, Bouvresse S, Bagot M, Wolkenstein P, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246–52.
Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40.
Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JK, et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174:1194–227.
Dorafshar AH, Dickie SR, Cohn AB, Aycock JK, O’Connor A, Tung A, et al. Antishear therapy for toxic epidermal necrolysis: an alternative treatment approach. Plast Reconstr Surg. 2008;122:154–60.
Shiga S, Cartotto R. What are the fluid requirements in toxic epidermal necrolysis? J Burn Care Res. 2010;31:100–4.
Kreymann KG, Berger MM, Deutz NEP, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on Enteral nutrition: intensive care. Clin Nutr. 2006;25:210–23.
Chronopoulos A, Pleyer U, Mockenhaupt M. Ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Klin Monatsbl Augenheilkd. 2012;229:534–9.
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53.
Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986;204:503–12.
Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007;87:144–8.
Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal Necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153:514–22.
Hirahara K, Kano Y, Sato Y, Horie C, Okazaki A, Ishida T, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. J Am Acad Dermatol. 2013;69:496–8.
Araki Y, Sotozono C, Inatomi T, Ueta M, Yokoi N, Ueda E, et al. Successful treatment of Stevens-Johnson syndrome with steroid pulse therapy at disease onset. Am J Ophthalmol. 2009;147:1004–11.
Schneck J, Fagot J‑P, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33–40.
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3.
Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: The University of Miami Experience. Arch Dermatol. 2003;139:39–43.
Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139:33–6.
Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil. 2004;25:246–55.
Faye O, Roujeau JC. Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IVIG). Clinical experience to date. Drugs. 2005;65:2085–90.
Lee HY, Lim YL, Thirumoorthy T, Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. Br J Dermatol. 2013;169:1304–9.
Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. 2012;167:424–32.
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847–53.
Arévalo JM, Lorente JA, González-Herrada C, Jiménez-Reyes J. Treatment of toxic epidermal necrolysis with Cyclosporin A. J Trauma. 2000;48:473–8.
Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71:941–7.
Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79:686–2.
González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporine use in epidermal Necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137:2092–100.
St John J, Ratushny V, Liu KJ, Bach DQ, Badri O, Gracey LE, et al. Successful use of Cyclosporin A for Stevens-Johnson syndrome and toxic epidermal Necrolysis in three children. Pediatr Dermatol. 2017;34:540–6.
Roujeau JC, Mockenhaupt M, Guillaume JC, Revuz J. New evidence supporting cyclosporine efficacy in epidermal necrolysis. J Invest Dermatol. 2017;137:2047–9.
Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586–9.
Kreft B, Wohlrab J, Bramsiepe I, Eismann R, Winkler M, Marsch WC. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904–6.
Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278–83.
Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF‑α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985–6.
Narita YM, Hirahara K, Mizukawa Y, Kano Y, Shiohara T. Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: Is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol. 2011;38:236–45.
Giudice G, Maggio G, Bufano L, Memeo G, Vestita M. Management of toxic epidermal Necrolysis with plasmapheresis and Cyclosporine A: our 10 years’ experience. Plast Reconstr Surg Glob Open. 2017;5:e1221.
Yang CW, Cho YT, Chen KL, Chen YC, Song HL, Chu CY. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal Necrolysis. Acta Derm Venereol. 2016;96:525–9.
Paulmann M, Kremmler C, Sekula P, Valeyrie-Allanore L, Naldi L, Kardaun S, et al. Long-term sequelae in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis: a 5-year analysis. Clin Transl Allergy. 2016;6(Suppl 3):31.
Kauppinen K. Cutaneous reactions to drugs. With special reference to severe bullous mucocutaneous eruptions and sulphonamides. A clinical study. Acta Derm Venereol Suppl. 1972;68:1–89.
Barbaud A, Collet E, Milpied B. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;3:555–62.
Ardern-Jones M, Mockenhaupt M. Making a diagnosis in severe cutaneous drug reactions. Curr Opin All Clin Immnunol. 2019;19:283–393.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Paulmann and M. Mockenhaupt declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Paulmann, M., Mockenhaupt, M. Severe skin reactions: clinical picture, epidemiology, etiology, pathogenesis, and treatment. Allergo J Int 28, 311–326 (2019). https://doi.org/10.1007/s40629-019-00111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-019-00111-8