Abstract
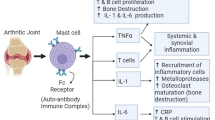
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that primarily affects the joints, with the main clinical manifestations being chronic, symmetrical, and peripheral multi-joint inflammatory lesions. Drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), disease-modifying anti-rheumatic drugs (DMARDs), and biologics play a very important role in the treatment of RA. Of these, the most commonly used are chemical drugs, such as NSAIDs, GCs, and DMARDs. In recent years, a number of new compounds have emerged for the treatment of RA, such as SYK inhibitors, JAK inhibitors, NSAID-CAI drugs, and Syk/PDGFR-α/c-Kit inhibitors. In this review, we summarize the most recently developed anti-RA chemical drugs and discuss the synthesis and biological activities of these various new compounds.














Similar content being viewed by others
References
Majithia V, Geraci SA (2007) Rheumatoid arthritis: diagnosis and management. Am J Med 120(11):936–939
Smolen JS, Aletaha D et al (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038
Singh J, Saag K, Bridges S et al (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68(1):1–26
Singh J, Wells G, Christensen R et al (2011) Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev (2):CD008794
Efthimiou P, Kukar M (2010) Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities. Rheumatol Int 30(5):571–586
Derksen V, Huizinga T, Van D (2017) The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol 39(4):437–446
Gao Q, Wang Y, Xu D et al (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:15
Alamanos Y, Drosos AA (2005) Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 4(3):130–136
Bottini N, Firestein G (2013) Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 9(1):24–33
Mor A, Abramson S, Pillinger M (2005) The fibroblast-likesynovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol 115(2):118–128
Gaffo A, Saag KG, Curtis JR (2006) Treatment of rheumatoid arthritis. Am J Health Syst Pharm 63(24):2451–2465
Oliveira R, Fierro I (2018) New strategies for patenting biological medicines used in rheumatoid arthritis treatment. Expert Opin Ther Pat 28(8):635–646
Singh G, Ramry D, Morfeld D et al (1996) Gastrointestinal tract complications of NSAID treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med 156(14):1530–1536
Pan T, Cheng T, Jia Y et al (2017) Anti-rheumatoid arthritis effects of traditional Chinese herb couple in adjuvant-induced arthritis in rats. J Ethnopharmacol 205:1–7
Ichikawa N, Yamanaka H (2012) Disease-modifying antirheumatic drugs. Clin Calcium 22(2):215–221
Raoof R, Willemen H, Eijkelkamp N (2018) Divergent roles of immune cells and their mediators in pain. Rheumatology (Oxford) 57(3):429–440
Ling S, Bluett J, Barton A (2018) Prediction of response to methotrexate in rheumatoid arthritis. Expert Rev Clin Immunol 14(5):419–429
Fox R, Herrmann M, Frangou C et al (1999) Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol 93(3):198–208
Plosker G, Croom K (2005) Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs 65(13):1825–1849
Walker K, Farrow S (2007) Rheumatoid arthritis. BMJ Clin Evid 8:1124–1169
Miller R, Petereit D, Sloan J et al (2016) N08C9 (Alliance): a phase 3 randomized study of sulfasalazine versus placebo in the prevention of acute diarrhea in patients receiving pelvicradiation therapy. Int J Radiat Oncol Biol Phys 95(4):1168–1174
Kirwan J, Bijlsma J, Boers M et al (2007) Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev (1):CD006356
Ethgen O, De Lemos Esteves F, Bruyere O et al (2013) What do we know about the safety of corticosteroids in rheumatoid arthritis. Curr Med Res Opin 29(9):1147–1160
Sharma J, Bhar S, Devi C (2017) A review on interleukins: the key manipulators in rheumatoid arthritis. Mod Rheumatol 27(5):723–746
Thakur S, Riyaz B, Patil A et al (2018) Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: an overview. Biomed Pharmacother 106:1011–1023
Wang L, Wang K, Chu X et al (2017) Intra-articular injection of Botulinum toxin A reduces neurogenic inflammation in CFA-induced arthritic rat model. Toxicon 126:70–78
Bally M, Dendukuri N, Rich B et al (2017) Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ 357:j1909
Buer J (2014) Origins and impact of the term ‘NSAID'. Inflammopharmacology 22(5):263–267
Machado G, Maher C, Ferreira P et al (2017) Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis 76(7):1269–1278
Derry S, Conaghan P, Da S et al (2016) Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 4:CD007400
Mallinson T (2017) A review of ketorolac as a prehospital analgesic. J Paramed Pract 9(12):522–526
Moore R, Derry S, Aldington D et al (2015) Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev (9):CD008659
Ashley P, Parekh S, Moles D et al (2016) Preoperative analgesics for additional pain relief in children and adolescents having dental treatment. Cochrane Database Syst Rev (8):CD008392
Eccleston C, Cooper T, Fisher E et al (2017) Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev 8:CD012537
Cooper T, Heathcote L, Anderson B et al (2017) Non-steroidal anti-inflammatory drugs (NSAIDs) for cancer-related pain in children and adolescents. Cochrane Database Syst Rev 7:CD012563
StarSurg C (2017) Safety of nonsteroidal anti-inflammatory drugs in major gastrointestinal surgery: a prospective, multicenter cohort study. World J Surg 41(1):47–55
Walsem A, Pandhi S, Nixon R et al (2015) Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther 17(1):66
Bally M, Dendukuri N, Rich B et al (2017) Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ 357:j1909
Auriel E, Regev K, Korczyn A (2014) Nonsteroidal anti-inflammatory drugs exposure and the central nervous system. Handb Clin Neurol 119:577–584
Fowler C (2017) The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol 152(5):594–601
Miguel Á (2015) Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 32(2):139–147
Kowalski L, Makowska J (2015) Seven steps to the diagnosis of NSAIDs hypersensitivity: how to apply a new classification in real practice. Allergy Asthma Immunol Res 7(4):312–320
Wang J (2015) Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis 44(2):385–396
Banti C (2016) Non-steroidal anti-inflammatory drugs (NSAIDs) in metal complexes and their effect at the cellular level. Eur J Inorg Chem 19:3048–3071
Häggström M, Richfield D (2014) Diagram of the pathways of human steroidogenesis. WikiJ Med 1(1):5
Liu C, Guan J, Kang Y et al (2010) Inhibition of dehydration-induced water intake by glucocorticoids is associated with activation of hypothalamic natriuretic peptide receptor-A in rat. PLoS One 5(12):e15607
Liu C, Chen Y, Kang Y et al (2011) Glucocorticoids improve renal responsiveness to atrial natriuretic peptide by up-regulating natriuretic peptide receptor-A expression in the renal inner medullary collecting duct in decompensated heart failure. J Pharmacol Exp Ther 339(1):203–209
Tarner I, Englbrecht M, Schneider M et al (2012) The role of corticosteroids for pain relief in persistent pain of inflammatory arthritis: a systematic literature review. J Rheumatol Suppl 90:17–20
Haywood A, Good P, Khan S et al (2015) Corticosteroids for the management of cancer-related pain in adults. Cochrane Database Syst Rev (4):CD010756
Chowdhury R, Naaseri S, Lee J et al (2014) Imaging and management of greater trochanteric pain syndrome. Postgrad Med J 90(1068):576–581
Mohamadi A, Chan J, Claessen F et al (2017) Corticosteroid injections give small and transient pain relief in rotator cuff tendinosis: a meta-analysis. Clin Orthop Relat Res 475(1):232–243
Banuelos J, Shin S, Cao Y et al (2016) BCL-2 protects human and mouse Th17 cells from glucocorticoid-induced apoptosis. Allergy 71:640–650
Massari F, Mastropasqua F, Iacoviello M et al (2012) The glucocorticoid in acute decompensated heart failure: Dr. Jekyll or Mr. Hyde. Am J Emerg Med 30(3):517.e5–517.e10
Gelber J (2017) CORR insights: corticosteroid injections give small and transient pain relief in rotator cuff tendinosis: a meta-analysis. Clin Orthop Relat Res 475(1):244–246
Buer J (2015) A history of the term DMARD. Inflammopharmacology 23(4):163–171
Smolen J, Heijde D, Machold K et al (2014) Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis 73(1):3–5
Nandi P, Kingsley G, Scott D (2008) Disease-modifying antirheumatic drugs other than methotrexate in rheumatoid arthritis and seronegative arthritis. Curr Opin Rheumatol 20(3):251–256
Mohammad S, Clowse M, Eudy A et al (2018) Examination of hydroxychloroquine use and hemolytic anemia in G6PDH-deficient patients. Arthritis Care Res 70(3):481–485
Harbut M, Vilcheze C, Luo X et al (2015) Auranofin exerts broad-spectrum bactericidal activities by targeting thio-redox homeostasis. Proc Natl Acad Sci USA 112(14):4453–4458
Park S, Lee J, Berek J et al (2014) Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53. Int J Oncol 45(4):1691–1698
Aggarwal R, Singh G, Kaushik P et al (2015) Molecular docking design and one-pot expeditious synthesis of novel 2,5-diarylpyrazolo[1,5-a] pyrimidin-7-amines as anti-inflammatory agents. Eur J Med Chem 101:326–333
Ligua H, Baoshun Z, You Y et al (2016) Synthesis and anti-inflammatory activity of paeonol analogues in the murine model of complete Freund’s adjuvant induced arthritis. Bioorg Med Chem Lett 26:5218–5221
Silvia B, Lorenzo D, Daniela V et al (2017) Design and synthesis of novel non steroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 60:1159–1170
Linhong H, Heying P, Tingxuan L et al (2017) Design and synthesis of a highly selective JAK3 inhibitor for the treatment of rheumatoid arthritis. Arch Pharm Chem Life Sci 350:e1700194
Maninder K, Manjinder S, Om S (2017) Oxindole-based SYK and JAK3 dual inhibitors for rheumatoid arthritis: designing, synthesis and biological evaluation. Future Med Chem 9(11):1193–1211
Ozlem A, Lorenzo D, Daniela V et al (2018) Discovery of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the management of rheumatoid arthritis. J Med Chem 61:4961–4977
Chieyeon C, Misuk J, Sunmin L et al (2018) Development of selective inhibitors for the treatment of rheumatoid arthritis: (R)-3-(3-(Methyl (7H-pyrrolo [2,3-d] pyrimidin-4-yl) amino) pyrrolidin-1-yl)-3- oxopropanenitrile as a JAK1-selective inhibitor. Bioorg Med Chem 26:1495–1510
Hisao H, Yasushi A, Ayako M et al (2018) Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem 26:4971–4983
Xiaokang L, Yahui H, Junfei C et al (2018) Discovery of novel Syk/PDGFR-α/c-Kit inhibitors as multi-targeting drugs to treat rheumatoid arthritis. Bioorg Med Chem 26:4375–4381
Romero-Estudillo I, Viveros-Ceballos JL, Cazares-Carreño O et al (2019) Synthesis of new α-aminophosphonates: evaluation as antiinflammatory agents and QSAR studies. Bioorg Med Chem 15;27(12):2376–2386
Acknowledgements
The Project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 2015-1098). The work was also supported by Chongqing Key Research Project of Basic Science and Frontier Technology (No. cstc2017jcyjBX0012), Foundation Project of Chongqing Normal University (No. 14XYY020), Chongqing General Research Program of Basic Research and Frontier Technology (No. cstc2015jcyjA10054), Chongqing Normal University Postgraduate’s Research and Innovation Project (No. YKC17004), the National Natural Science Foundation (21662012, 41866005), Postgraduate Research and Innovation Project of Hainan Normal University (Hsyx2018-8), and Open Foundation Project of Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education (RDZH2019002), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, S., Zou, H., Chen, G. et al. Synthesis and Biological Activities of Chemical Drugs for the Treatment of Rheumatoid Arthritis. Top Curr Chem (Z) 377, 28 (2019). https://doi.org/10.1007/s41061-019-0252-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-019-0252-5