Clinically, the metabolic syndrome involves a cluster of disturbances in which glucose intolerance represents an important symptom. Metabolic syndrome diagnosis implies in positive results to at least three metabolic alterations including insulin resistance, hypertension, obesity, endothelial dysfunction and blood lipid profile alterations(Reference Reaven1, Reference Zecchin, Carvalheira and Saad2). These multiple risk factors accelerate the incidence of CVD in a cooperative way(Reference Reaven1–Reference Kaplan4).
Obesity prevalence has quadrupled in the past 25 years in the USA; 16 % of children and 30 % of adults are now affected and many of these obese individuals suffer from the metabolic syndrome(Reference Nakagawa, Tuttle, Short and Johnson5). Projections estimate that, in the year 2010, there will exist 50–75 million individuals with manifestation of this syndrome in this country alone(Reference Samad, Uysal, Wiesbrock, Pandey, Hotamisligil and Loskutoff6).
The increased fructose consumption in contemporaneous Western society has been associated with the high prevalence of non-alcoholic fatty liver disease (NAFLD)(Reference Cave, Deaciuc, Mendez, Song, Joshi-Barve, Barve and McClain7), obesity, type 2 diabetes and metabolic syndrome so far(Reference Gross, Li, Ford and Liu8, Reference Astrup and Finer9). In fact, laboratory animals fed on fructose-rich diets show glucose intolerance, insulin resistance, hyperinsulinaemia and dyslipidaemia(Reference Hwang, Ho, Hoffman and Reaven10). A high flux of fructose to the liver perturbs glucose metabolism and glucose uptake pathways, and leads to a significantly enhanced rate of lipogenesis and TAG synthesis, driven by the high flux of glycerol and acyl portions of TAG molecules from fructose catabolism(Reference Katakam, Ujhelyi, Hoenig and Miller11–Reference Hallfrisch13). These metabolic disturbances maybe underlie the induction of insulin resistance, commonly observed with high fructose feeding in both human subjects and animal models(Reference Basciano, Federico and Adeli14). Since fructose-fed rats reveal signs of the metabolic syndrome, they are used as an experimental model of the human condition(Reference Okada, Yoshino, Takeuchi, Endoh, Ohta, Jinno, Yokoyama, Izawa and Kobayshi15).
The metabolic alterations observed in fructose-fed rats are quite divergent among the studies, probably due to study design. Differences between studies include: the strain of rat used, such as Wistar(Reference Joyeux-Faure, Rossini, Ribuot and Faure16, Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17) and Sprague–Dawley(Reference Lee, Ko, Hsu and Ho18, Reference Sánchez-Lozada, Tapia and Jiménez19); the amount and route of fructose administration – diet (60 %)(Reference Joyeux-Faure, Rossini, Ribuot and Faure16, Reference Sánchez-Lozada, Tapia and Jiménez19), oral administration (8 g/kg)(Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17) or drinking water (10 %)(Reference Sánchez-Lozada, Tapia and Jiménez19); the age of the animals at the beginning of the experiment – young(Reference Joyeux-Faure, Rossini, Ribuot and Faure16, Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17) or adults(Reference Sánchez-Lozada, Tapia and Jiménez19). Also, the period of fructose administration applied has been non-standard: from 4(Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17), 6(Reference Lee, Ko, Hsu and Ho18) or 8 weeks(Reference Sánchez-Lozada, Tapia and Jiménez19) or during several months(Reference Lee, Ko, Hsu and Ho18). Natural variation of the control diet is another potential problem of animal studies. It can be a particular problem with non-purified diets, but the use of synthetic diets is largely described in the literature(Reference Daly, Vale, Walke, George, Albert and Mathers20). Therefore, different experimental designs for fructose administration in rats induce variable degrees of physiological responses.
In the search of an adequate experimental model to simulate the human metabolic syndrome, the present study was designed to analyse the metabolic characteristics of Wistar rats submitted to high fructose ingestion by different protocols.
Methods
Animal care and experimental design
Male Wistar rats were obtained from the São Paulo State University (UNESP Central Bioterium, Botucatu Campus, SP, Brazil). The rats were kept in a room with the temperature set to 25 ± 1 °C and with a photoperiod of 12 h–12 h at the Physical Education Department Biodynamic laboratory of UNESP (Rio Claro campus, São Paulo, Brazil). Free access to water and food was provided. All experiments were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe no. 123, Strasbourg 1985).
The animals were separated at random and the studies were carried out in three separated series of experiments. In the first, two groups of adult rats were studied: the control group (C1; n 6) received regular rodent chow (57·3 % carbohydrate, 41·2 % as starch, Labina; Purina, São Paulo, Brazil) and the fructose group (F1; n 6) was fed on a regular rodent chow and 10 % of drinking water was composed of fructose solution. Both groups started the experiment aged 90 d and the follow-up was done for 8 weeks.
In the second experiment, two groups of adult rats were also evaluated: the control group (C2; n 6) was fed on a purified balanced diet (AIN-93G)(Reference Reeves, Nielsen and Fahey21) and a fructose group (F2; n 6) was fed on a purified 60 % fructose diet (Table 1). Both groups started the experiment at age 90 d and the follow-up was done for 4 weeks.
Table 1 Composition of the balanced and fructose-rich diets
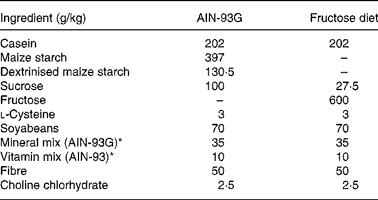
* See Reeves et al.(Reference Reeves, Nielsen and Fahey21).
Two groups of young rats were analysed in the third experiment: the control group (C3; n 6) was fed on a balanced AIN-93G diet and the fructose group (F3; n 6) was fed on a 60 % fructose diet. Both groups started the experiment at age 28 d and the follow-up was done for 8 weeks.
All animals were weighed and measured (nose to anus length) once per week. At the end of the experimental period, the Lee index was calculated(Reference Bernardis and Peterson22) (by dividing the cubic root of the final body weight (g) by the final body length (cm) and multiplying by 1000). This index for rats is equivalent to the human BMI.
The vessels containing the diets and the bottle of water were refilled each 2 d. Once per week, the differences between the full vessels or full bottles and the content 24 h later were considered as the amount consumed and were registered. The average amount of fructose ingested was calculated as 60 % of diet (experiments 2 and 3) or 10 % of the water ingested during the whole period (experiment 1).
Oral glucose tolerance test
At the end of each experiment, the rats were fasted for 15 h. Glucose was administered into the stomach of the rats through a gastric catheter at the final dose of 2·0 g/kg body weight. Blood samples for serum glucose determination were obtained from a cut at the tip tail at 0, 30, 60 and 120 min. Serum glucose determination was made by the glucose oxidase method (Laborlab Kit; Guarulhos, SP, Brazil)(Reference Latorraca, Carneiro, Boschero and Mello23). The glycaemic response during the oral glucose tolerance test was evaluated by the total area under the serum glucose curve using the trapezoidal method(Reference Mathews, Altaman, Campbell and Royston24).
Subcutaneous insulin tolerance test
At the end of all experimental series, subcutaneous insulin tolerance tests were performed for peripheral insulin sensitivity evaluation. The insulin tolerance tests consisted of a bolus injection of regular insulin at the dorsal region (30 mU/g body weight). Blood samples were obtained from a cut at the tip tail at 0, 30, 60 and 120 min for serum glucose determination by the glucose oxidase method (Laborlab Kit)(Reference Latorraca, Carneiro, Boschero and Mello23). A constant for serum glucose disappearance (Kitt) was calculated from the formula 0·693/t1/2. The serum glucose t1/2 was calculated from the slope of the least square analysis of serum glucose concentration from 0–30 min after insulin injection, when serum glucose concentration decreased linearly(Reference Lundbaek25).
Blood chemistry and body fat
Because of multiple aspects involving insulin sensitivity evaluation, the fasting insulin concentration was also assessed.
All animals were killed by decapitation and trunk blood was collected for serum TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and glucose determinations by colorimetric procedures(Reference Nogueira, Strufaldi, Hirata, Abdalla and Hirata26). Retroperitoneal, mesenteric (visceral) and subcutaneous posterior fat depots were excised and weighed according to Cinti(Reference Cinti27). Liver total lipid concentration(Reference Nogueira, Strufaldi, Hirata, Abdalla and Hirata26) was also determined.
Statistics
In each experiment, the values of weight and body length throughout the period of dietary intake were analysed by two-way ANOVA. All the other results were analysed statistically by the unpaired Student's t test. A level of 5 % was taken for statistical significance.
Results
The weight and body-length values in the first experiment are shown in Fig. 1. No difference was revealed.

Fig. 1 Weight (a) and length (b) of fructose-fed (–▲–) and control (–●–) animals in the first experiment (P>0·05).
Data from the first series are displayed in Table 2. No metabolic difference was observed between control (C1) and fructose-fed (F1) rats in any of the parameters evaluated. The average amount of fructose ingested corresponded to 1·74 g/100 g body weight per d.
Table 2 Effects of 8 weeks of fructose feeding as 10 % solution in drinking water in adult male Wistar rats (experiment 1)*
(Mean values and standard deviations of six animals per group)

GTT, glucose tolerance test; Kitt, constant for serum glucose disappearance; bw, body weight.
* See Methods for details of the diets.
No differences were observed in weight or body-length values in the second experiment (Fig. 2).

Fig. 2 Weight (a) and length (b) of fructose-fed (–▲–) and control (–●–) animals in the second experiment (P>0·05).
The data of the second experiment are shown in Table 3. The fructose group (F2) presented higher values for the area under the serum glucose curve during glucose tolerance tests when compared with the control group (C2). Rats of the F2 group developed insulin resistance when compared with the C2 group, indicated by a lower constant for serum glucose disappearance (Kitt) value and a higher fasting insulin concentration. No difference was observed in the basal serum glucose values between the two groups. Serum total cholesterol, HDL, LDL and TAG were higher in the F2 group than in the C2 group. In this experiment higher retroperitoneal, mesenteric and subcutaneous fat depot weights were observed in the F2 group in comparison with the C2 group. The concentration of liver total lipids was also higher in the F2 group compared with the C2 group. No difference was found between the groups in the results related to the Lee index. The average amount of fructose ingested in this second experiment was 2·70 g/100 g body weight per d.
Table 3 Effects of 4 weeks of feeding a 60 % fructose diet in adult male Wistar rats (experiment 2)†
(Mean values and standard deviations of six animals per group)

GTT, glucose tolerance test; Kitt, constant for serum glucose disappearance; bw, body weight.
* Mean value was significantly different from that of the control group (P < 0·05; unpaired t test).
† For details of the diets, see Methods.
No difference was observed in weight or body-length values in the third experiment (Fig. 3).

Fig. 3 Weight (a) and length (b) of fructose-fed (–▲–) and control (–●–) animals in the third experiment (P>0·05).
Table 4 shows data from the third series of experiments. The area under the serum glucose curve during glucose tolerance tests and TAG concentration were higher for the F3 group when compared with the C3 group. No differences were observed between the groups in insulin resistance, serum total cholesterol, basal glucose, HDL, LDL, retroperitoneal, mesenteric and subcutaneous fat depot weights and the Lee index. In this third experiment, the average amount of fructose ingested was 3·75 g/100 g body weight per d.
Table 4 Effects of 8 weeks of feeding a 60 % fructose diet in young male Wistar rats (experiment 3)†
(Mean values and standard deviations of six animals per group)

GTT, glucose tolerance test; Kitt, constant for serum glucose disappearance; bw, body weight.
* Mean value was significantly different from that of the control group (P < 0·05; unpaired t test).
† For details of the diets, see Methods.
Discussion
There has been clinical and epidemiological evidence suggesting a progressive association of metabolic syndrome development and high fructose consumption(Reference Elliott, Keim, Stern, Teff and Havel28). Indeed, a marked increase in obesity and metabolic syndrome prevalence has been linked to a 30 % overall increase in fructose ingestion in the last 20 years within the USA. It has been associated with the introduction of high-fructose maize syrup as a sweetener in soft drinks and other foods(Reference Nakagawa, Tuttle, Short and Johnson5). In the present study, we examined the effect of three different protocols of fructose administration on the metabolic characteristics of Wistar rats.
In the first protocol, fructose (10 %) was administered to adult (90 d) male Wistar rats in drinking water during 8 weeks. No metabolic differences were observed between the control and fructose-fed rats. Using the same procedure, Sanchez-Lozada et al. (Reference Sánchez-Lozada, Tapia and Jiménez19) reported that fructose administration was able to induce systemic hypertension, hyperuricaemia and hypertriacylglycerolaemia in adult male Sprague–Dawley rats. Roglans et al. (Reference Roglans, Vilà, Farré, Alegret, Sánchez, Vázquez-Carrera and Laguna29) reported hypertriacylglycerolaemia and hepatic steatosis in Sprague–Dawley rats (the authors neither mention the age nor the weight of the animals at the beginning of the experiment) that had received fructose (10 %) in drinking water for 2 weeks. Additionally, while adult Sprague–Dawley rats fed fructose through drinking water developed features of the metabolic syndrome, adult Wistar rats presented a serum biochemical profile considered to be healthier for the cardiovascular system. Thus, the reported differences may be associated with the rat lineage used. In fact, Wistar rats seem to be less affected by the deleterious effects of fructose when administered in drinking water than Sprague–Dawley ones.
Sanchez-Lozada et al. (Reference Sánchez-Lozada, Tapia and Jiménez19) compared the metabolic effects of fructose (10 %) in drinking water and a high-fructose (60 %) diet in Sprague–Dawley rats. Both procedures induced hyperuricaemia and hypertriacylglycerolaemia. However, the 60 % fructose diet resulted in a higher fructose energy intake, which was directly associated with worsening metabolic syndrome parameters. Other studies have also reported hyperinsulinaemia, hypertriacylglycerolaemia and glucose intolerance in Sprague–Dawley rats fed on 60 % fructose diets from 4 to 7 weeks(Reference Katakam, Ujhelyi, Hoenig and Miller11, Reference Kelley, Allan and Azhar12, Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17, Reference Lee, Ko, Hsu and Ho18, Reference Oron-Herman, Rosenthal, Mirelman, Miron, Rabinkov, Wilchek and Sela30–Reference Wang, Hattori, Satoh, Iwata, Banba, Monden, Konsuke, Kamikawa and Kasai32). Thus, in the second experiment series, a fructose diet was administered to adult male Wistar rats (age 90 d) for 4 weeks.
Using the fructose-rich diet protocol, we also succeeded in inducing signs of the metabolic syndrome in fructose-fed Wistar rats, such as glucose intolerance, insulin resistance and high serum TAG, total cholesterol and LDL as well as a high concentration of total liver lipids.
The elevated liver lipid accumulation is the sign of NAFLD that may contribute to the development of non-alcoholic steatohepatitis(Reference Pitt33) in individuals who do not consume significant amounts of ethanol(Reference Zivkovi, Germam and Sanyal34). This result reinforces the assumption that not only high-fat dietary consumption plays a role in NAFLD, but also high fructose ingestion. In this way, Ouyang et al. (Reference Ouyang, Cirillo, Sautin, McCall, Bruchette, Diehl, Johnson and Abdelmalek35) showed that fructose ingestion in patients with NAFLD was 2- to 3-fold higher than in control groups.
It has been reported that adult Sprague–Dawley rats given fructose solution with standard diets gained more weight and had significantly more fat tissue weight than control rats given only a standard diet(Reference Kanarek and Orthen-Gambill36). Alterations in body fat were also observed in the fructose-fed rats of the second experiment, which showed retroperitoneal, mesenteric and subcutaneous fat depot weight increase.
Lau et al. (Reference Lau, Faerch, Glümer, Tetens, Pedersen, Carstensen, Jorgensen and Borch-Johnsen37), in a prospective study with non-diabetic patients, found no association between the glycaemic index or glycaemic load of the diet with the probability of developing insulin resistance. Barbosa et al. (Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17) compared the effects of glucose and fructose supplementation (8 g/kg) in Wistar rats during 3 weeks. The authors reported insulin resistance and hypertriacylglycerolaemia in response to both treatments, but the glycaemia was higher in the fructose group. The fructose glycaemic index is about 3-fold lower than glucose and therefore the alterations observed may not be related to this index or even to glycaemic load (glycaemic index multiplied by amount in grams). In addition, the second experiment, which revealed more damage to the rats, presented just an intermediate value of daily fructose ingested in comparison with the first and third experiments.
The third experiment series was designed to analyse the metabolic alterations caused in young (28 d) Wistar rats by administration of a high-fructose diet for 8 weeks. The young animals fed on the high-fructose diet showed hypertriacylglycerolaemia and glucose intolerance in the absence of alterations in insulin sensitivity, serum total cholesterol, LDL and HDL concentration as well as mesenteric, retroperitoneal and subcutaneous fat depot weights when compared with the control group. In contrast, insulin sensitivity impairment has been described in 5-week-old Wistar–Hannover rats, after 28 d of high-fructose diet ingestion associated with hypertriacylglycerolaemia and unchanged body-weight gain, basal serum glucose and serum total cholesterol(Reference Bezerra, Ueno, Silva, Tavares, Carvalho, Saad and Gontijo38).
Interestingly, the third experiment showed that the average amount of daily fructose ingestion was almost 40 % higher than in the second experiment, but did not induce a complete metabolic syndrome picture in these animals. A reasonable hypothesis could be the influence of protective mechanisms linked to youth in Wistar rats. However, further investigation will be necessary.
It is important to note that the AIN-93G provided in both series of experiments is recommended for the growth phase and is composed of 75 % more fat and 42·9 % more casein than an adult maintenance diet. However, the C2 rats presented fat depot values very similar to the C1 rats that ingested the regular rodent chow.
The dietary ingestion of fructose in humans is basically associated with soft drinks (whose sugars are composed of 45 % of glucose and 55 % of fructose), which account for just 8 % of total energy intake(Reference Park and Yetley39). However, Montonen et al. (Reference Montonen, Järvinen, Knekt, Heliövaara and Reunanen40) in a cohort-designed study reported that the risk of type 2 diabetes occurrence is 1·67 higher with this dietary profile. The emerging evidence from recent epidemiological and biochemical studies clearly suggests that the high fructose dietary intake has rapidly become an important causative factor in the development of the metabolic syndrome(Reference Gross, Li, Ford and Liu8).
Nevertheless, the present study was not designed to investigate fructose dose-response characteristics, as there are different metabolic responses to high-fructose diets even within rodents(Reference Barbosa, Albuquerque, Faria, Oliveira and Castilho17). The importance of the present study resides in identifying the most adequate protocol to induce the metabolic syndrome in Wistar rats. According to the present findings, feeding adult rats (90 d) on a purified high-fructose (60 %) diet for 4 weeks seems to be an appropriate protocol for this purpose.
Concerning the limitations of the study, the lack of baseline values for the biochemical variables implies that already existent differences could have been missed. However, the randomisation process to compose the groups diminishes this possibility.
The absence of energy expenditure or physical activity measures does not exclude the possibility that the differences in the fat depots are related to lower physical or metabolic activity. Nonetheless, based on the randomisation assumption it would imply that the fructose diet plays a role in spontaneous physical activity. Additional studies may answer this question.
In conclusion, the results in the three series of experiments reveal that high fructose intake is more effective to produce signs of the metabolic syndrome in adult than in young Wistar rats, despite a shorter feeding period. Also, diet seems to provide a better effective route for fructose administration than drinking water.
Acknowledgements
The authors thank Clarice Y. Sibuya, Eduardo Custódio and José Roberto R. Silva for technical assistance. This research was supported by Brazilian foundations FAPESP (process: 07/54 098-0 and 05/57 741-6) and CNPQ (process: 30 027/2004-6). R. F. M was responsible for the statistical analysis and wrote the manuscript. C. R. was responsible for the biochemical analysis and was co-author of the manuscript. J. A. O. conducted study 1. E. S. was responsible for designing and planning study 1. M. A. R. M. designed, planned and oversaw studies 2 and 3. All the authors read and agreed with the final format. The study authors have no conflict of interests.











