Malnutrition is a major public health problem throughout the developing world(Reference Müller and Krawinkel1). Lack of protein-energy is the most lethal form of malnutrition(2). Maternal malnutrition during lactation contributes to the process of metabolic programming, altering, for instance, the number and size of adipocytes and consequently the serum concentration and action of adipocytokines, such as leptin, adiponectin and resistin, inducing changes in the glucose metabolism and insulin action(Reference Fagundes, Moura and Passos3, Reference Passos, Vicente and Lisboa4). Type 2 diabetes mellitus, hypertension and dyslipidaemia that are components of the metabolic syndrome are associated with poor early growth, especially on gestation(Reference Barker, Hales and Fall5, Reference Ozanne, Lewis and Jennings6). Malnutrition during lactation is associated with the programming of the thyroid and growth hormone function that can compromise body weight and length(Reference Passos, Ramos and Dutra7, Reference Moura, Lisboa and Custódio8).
One of the strategies for the treatment of childhood malnutrition is to increase energy density of foods and is often achieved by increasing the lipid content(Reference Dutra de Oliveira and Rolando9), especially using vegetable oils(10). This adequate offer of energy is indispensable during the recovery phase and has stimulating the catch-up effect as the main objective. The catch-up is considered a physiological adaptation that allows man and animals to return to their genetically programmed growth trajectory after a period of growth retardation(Reference Crescenzo, Samec and Antic11). The catch-up is dependent on the amount of food, the initial hyperphagia, the efficiency of the utilization of energy, the different distribution of body fat and the type of dietary fat(Reference Soriguer, Moreno and Rojo-Martínez12). This catch-up phenomenon has been associated with the programming of a thrifty phenotype, by several authors(Reference Bieswal, Ahn and Reusens13–Reference Prentice and Moore16). The programming of the thyroid function by maternal malnutrition during lactation can compromise the catch-up phenomenon(Reference Passos, Ramos and Dutra7). Neonatal malnutrition programmes for some metabolic syndrome features later in life. The recovery of malnutrition with high-fat diet was studied with different diets. A recent study(Reference Desai, Babu and Ross17) showed, for example, an impairment of glucose homeostasis in male rats whose mothers were food restricted during lactation, and this is aggravated by a diet enriched with condensed milk.
Despite the knowledge about the importance of maintaining a low ratio of n-6/n-3 fatty acid for health, there are few reports focusing on the recovery from malnutrition after critical periods of development using higher energy-density diets, enriched with vegetable oil containing PUFA and MUFA after weaning. In Brazil, the population consumption of soya oil (ratio n-6/n-3 = 6·75) represents 82 % of the energy originating from fat sources, while rapeseed oil (ratio n-6/n-3 = 1·90) represents less than 4 %(Reference Levy-Costa, Sichieri and Pontes18, Reference McDonald19). So, the aim of this study is to evaluate the offspring's response to maternal malnutrion during lactation, and the treatment with a high-fat diet constituted with vegetable oils, comparing those rich in PUFA or MUFA, on its ability to programming body weight, fat body distribution, lipid profile, and leptin and thyroid hormone serum concentration on young adult animals.
Experimental methods
Wistar rats were kept in a room with controlled temperature (25 ± 1°C) and with an artificial dark–light cycle (lights on from 07.00 to 19.00 hours). Virgin female rats (3 months old) were caged with male rats and after mating each female was placed in an individual cage with free access to water and food until delivery.
Within 24 h of birth (day 0), excess pups were removed so that only six male pups were kept per dam (n 24), as it has been shown that this procedure maximizes lactation performance(Reference Fishbeck and Rasmussen20). During 21 d of lactation, rats dams were continued on an ad libitum diet (control group, C; n 6) of standard laboratory food (Agroceres®, São Paulo) or a 50 % food restricted (FR group, six per group). Six litters were used per group and two animals of each litter were randomly assigned to each group.
After weaning (day 21), control group (n 12) and some of the undernourished litters (n 12) were fed with purified ration AIN-93G(Reference Reeves21) containing 7 g/100 g ration of soya oil (7 %sC and 7 %sFR, respectively). The remaining undernourished rats (n 24) received the same purified diet, however, containing 19 g/100 g ration of soya oil (19 %sFR, n 12) or rapeseed oil (19 %cFR, n 12). Both C and FR rats received the same amounts of vitamins and minerals per g ration (Table 1). Food intake, body mass (g) and length (cm), and body density (g/cm)(Reference Duffy, Lewis and Mayhugh22) were evaluated in all pups every 3 d.
Table 1 Composition of ration used for normal and food-restricted animals
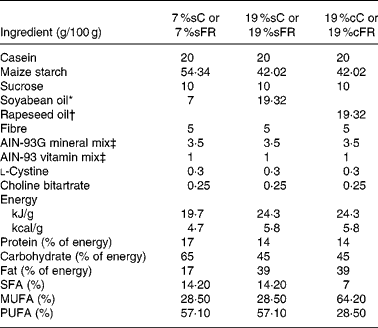
7 %sC, normal control group fed with 7 % soya oil; 7 %sFR, food-restricted group fed with 7 % soya oil; 19 %cC, normal control group fed with 19 % rapeseed oil; 19 %cFR, food-restricted group fed with 19 % rapeseed oil; 19 %sC, normal control group fed with 19 % soya oil; 19 %sFR, food-restricted group fed with 19 % soya oil.
* Commercial soyabean oil, Liza®.
† Commercial rapeseed oil, Salada Especial®.
‡ Formulated to meet the American Institute of Nutrition AIN-93G recommendation for rodent diets(Reference Reeves21).
At 60 d, after 8 h of fasting, a blood sample was collected from the tail tip to determine glucose basal serum concentration, using a reagent strip (Accu-Chek Advantage; Roche). Then the rats were killed by decapitation. Blood was collected for posterior assays of leptin (ng/ml), thyroxine (μg/l) and triiodothyronine (ng/l) serum concentrations by RIA using commercial kits (Linco Research, St Charles, MO, USA for leptin; MP Biomedicals Inc., New York, USA for thyroxine and triiodothyronine). For leptin, the inter-assay and intra-assay CV were 3·1 and 4·2 %, respectively, and the limit of detection was 0·04 ng/100 ml. For thyroid hormones the intra-assay CV were 4·5 % for triiodothyronine and 4·0 % for thyroxine. Serum concentrations of albumin (mg/l), TAG (mg/l), cholesterol (mg/l) and HDL-cholesterol (mg/l) were determined by a colorimetric method (Bioclin, Belo Horizonte, MG, Brazil).
Heart, brain, liver, testis, kidneys and visceral fat mass were weighed. Masses are expressed as total (g) organ mass and the fractional (g/100 g) mass (adjusted to total body mass). Body fat was measured by the Leshner method(Reference Leshner, Litwin and Squibb23) and expressed by the rate of carcass body mass and carcass fat content.
To understand better the effects of a diet high in MUFA or PUFA on body development we used normal control animals fed on these same diets, to compare with the malnourished rats, during weaning. Male Wistar rats were randomized after weaning (day 21) to receive either a control diet (containing 7 g soya oil and 54 g maize starch/100 g; 7 %sC group, n 11) or a high-fat diet (containing 19 g soya or rapeseed oil and 42 g maize starch/100 g; 19 %sC group, n 12 and 19 %cC group, n 12, respectively). Food intake, body mass (g) and length (cm) were evaluated every 3 d from weaning until death, when the rats were 60 d old. Blood samples were collected and serum concentrations of TAG (mg/l), cholesterol (mg/l), HDL-cholesterol (mg/l) were determined, as described earlier.
The use and handling of experimental animals followed the principles described in the guide for the care and use of laboratory animals(Reference Bayne24).
For statistical analyses we used the Graph Pad Prism statistical package version 4.02 (San Diego, CA, USA). Body mass and length at weaning (day 21) were compared by the Student's t test. After weaning, food intake, body mass and length, and body density were analysed by two-way ANOVA, followed by Bonferroni post-test. The remaining results were analysed by one-way ANOVA, followed by Newman–Keuls post-test. Differences were considered significant at P < 0·05. The results remain the same, when we included the dams as a blocking factor in the ANOVA analysis.
Results
Normal control animals treated with MUFA- or PUFA-enriched diets showed no difference among the groups for food intake (Fig. 1 (A)). Meanwhile, body weight gain did not differ until day 33 but, after that, high-fat groups gained more weight than controls (P < 0·05; Fig. 1 (B)). Also, body length was higher for the high-fat groups, after day 24 (P < 0·05; Fig. 1 (C)). High-fat groups showed an abdominal fat mass higher than controls (19 %sC, 47 %; 19 %cC, 39 %; P < 0·05; Fig. 2). High-fat groups showed lower TAG ( − 38 %, P < 0·05) and higher HDL-cholesterol (+35 %, P < 0·05) serum concentrations, when compared to controls, while total cholesterol did not differ among the groups (Table 2).

Fig. 1 Food intake (A), body mass (B) and body length (C) post-weaning until 60 d old, of normal rats fed with control diet with 7 % soya oil (○, 7 %sC, n 11) or with high-fat diet containing 19 % soya (△, 19 %sC, n 12) or 19 % rapeseed oil (●, 19 %cC, n 12). Values are means. * Mean values for 19 %sC and 19 %cC were significantly different from those of the 7 %sC group (two-way ANOVA; P < 0·05).

Fig. 2 Food intake post-weaning until 60 d old, of the control (○, 7 % soya oil, 7 %sC, n 12) and the undernourished litters (food-restricted (FR) groups fed with ration containing 7 % soya oil (●, 7 %sFR, n 12) or containing 19 % soya oil (△, 19 %sFR, n 12) or 19 % rapeseed oil (▲, 19 %cFR, n 12). Values are means. a,b Mean values at a time-point with unlike superscript letters were significantly different (two-way ANOVA; P < 0·05).
Table 2 Serum analyses of normal groups, at 60 d old†
(Mean values with their standard errors)
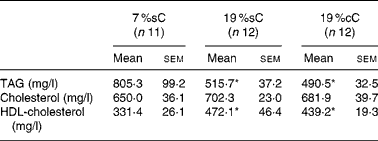
7 %sC, normal control group fed with 7 % soya oil; 19 %cC, normal control group fed with 19 % rapeseed oil; 19 %sC, normal control group fed with 19 % soya oil.
* Mean values were significantly different from those of the 7 %sC group (one-way ANOVA; P < 0·05).
† For details of procedures and diets, see Experimental methods.
At weaning, FR groups showed significantly lower body mass (19·05 (sem 0·48) g) and length (14·7 (sem 0·15) cm) compared to the C group (36·63 (sem 1·68) g; 18·58 (sem 0·30) cm), when the animals were 21 d old.
After weaning, 19 %sFR and 19 %cFR groups showed similar food intake between them and significantly lower than their C group, after 36 d old (Fig. 2), while the values of 7 %sFR were intermediary but not significantly different from C and FR-treated groups. During all experimental treatment, FR groups showed a significantly lower growth gain compared to the C group. Beside, 19 %sFR and 19 %cFR groups showed a significantly lower body mass and length gain compared to the 7 %sFR group, after 36 and 42 d, respectively. In regard to body density, similar results were observed after 39 d (Fig. 3).

Fig. 3 Body mass (A), length (B) and density (C) post-weaning until 60 d old, of the control (○, 7 % soya oil, 7 %sC, n 12) and the undernourished litters (food-restricted (FR) groups) fed with ration containing 7 % soya oil (●, 7 %sFR, n 12) or containing 19 % soya oil (△, 19 %sFR, n 12) or 19 % rapeseed oil (▲, 19 %cFR, n 12). Values are means with their standard errors depicted by vertical bars. a,b,c Mean values at a time-point with unlike superscript letters were significantly different (two-way ANOVA; P < 0·05).
The groups did not show significant difference in glucose, albumin, TAG, cholesterol, leptin and triiodothyronine serum concentrations when they were 60 d old. The FR high-fat treated groups showed a significantly higher HDL-cholesterol compared to C and 7 %sFR groups. Thyroxine plasma level was significantly lower in 19 %sFR compared to the 7 %sFR group, while the 19 %cFR group presented an intermediary mean value (Table 3).
Table 3 Serum analyses of food-restricted groups, at 60 d old*
(Mean values with their standard errors)
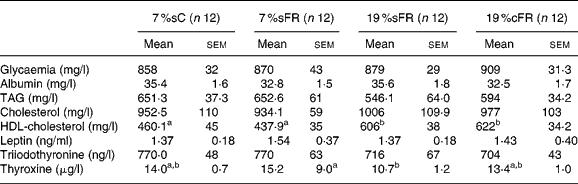
7 %sC, normal control group fed with 7 % soya oil; 7 %sFR, food-restricted group fed with 7 % soya oil; 19 %cFR, food-restricted group fed with 19 % rapeseed oil; 19 %sFR, food-restricted group fed with 19 % soya oil.
a,b Mean values within a row with unlike superscript letters were significantly different (one-way ANOVA; P < 0·05).
* For details of procedures and diets, see Experimental methods.
At 60 d old, total wet organ mass (brain, heart, kidneys and testis) was significantly lower in FR groups than the C group. 19 %cFR heart was significantly lower compared to other groups. 19 %sFR and 19 %cFR kidneys and testis were significantly lower than the 7 %sFR group. However, when the organ mass was expressed relative to body mass, 19 %sFR and 19 %cFR brain, heart and liver were higher compared to the C group; and 19 %cFR brain, liver and testis were higher than all other groups (Table 4).
Table 4 Body organs and adiposity analyses of food-restricted groups, at 60 d old*
(Mean values with their standard errors)

7 %sC, normal control group fed with 7 % soya oil; 7 %sFR, food-restricted group fed with 7 % soya oil; 19 %cFR, food-restricted group fed with 19 % rapeseed oil; 19 %sFR, food-restricted group fed with 19 % soya oil.
a,b Mean values within a row with unlike superscript letters were significantly different (one-way ANOVA; P < 0·05).
* For details of procedures and diets, see Experimental methods.
Groups fed with high-fat diet showed significantly lower absolute and relative visceral fat mass content and body fat mass compared to the C group. No significant differences were observed between the 7 %sFR and C groups (Table 4).
Discussion
It is well known that maternal malnutrition causes profound changes to milk composition, which impairs body weight gain in pups(Reference Passos, Ramos and Moura25). Neonatal malnutrition is associated with several features of the metabolic syndrome later in life(Reference Moura and Passos14). Nevertheless, there are few reports focusing on recovery treatment from early life malnutrition, especially with high-fat diets(Reference Ozanne, Lewis and Jennings6, Reference Desai, Babu and Ross17, Reference Vickers, Reddy and Ikenasio26). In the present study it was observed that the animals whose mothers were FR on lactation did not recover their lower length and body weight, nor body density, although they were treated with a high-fat diet after weaning until they were 60 d old. This, in part, is explained by the lower food intake that those animals presented, and could be due to a satiety effect of the vegetable oils(Reference Harrold, Widdowson and Clapham27, Reference Prentice and Doppitt28). However, other studies have reported that in adult life FR animals, fed a normal diet, gain more weight than the controls(Reference Moura, Lisboa and Custódio8, Reference Desai, Babu and Ross17, Reference Vickers, Reddy and Ikenasio26, Reference Teixeira, Passos and Ramos29).
High-fat diet during gestation and lactation is associated with higher total and visceral fat in adult offspring, caused by an increase of orexigenic neuropeptides and higher food intake(Reference Beck, Kozak and Moar30). Also, a higher-fat diet after weaning was associated with higher visceral and total fat mass in the adult offspring(Reference Ghibaudi, Cook and Farley31). This association was corroborated by the present results, when normal animals are treated with enriched oil diets. Conversely, when the animals were imprinted by a maternal FR diet, the enriched oil diet induced a lower visceral fat mass, suggesting that the programming effect of maternal malnutrition during lactation affects the response of those animals to a high-fat diet, when they were young. In spite of the lower fat mass, presented in the 19 % FR rats, leptin serum concentration did not alter. We can suggest some possibilities for this unaltered serum concentration: control animals already had a leptin serum concentration that did not decrease any further with leanness; subcutaneous fat mass can be produced sufficiently to maintain leptin; or the metabolic clearance of leptin is decreased.
It has been shown in some studies that undernutrition at critical development periods causes reduction in organ growth and permanent changes in their metabolism and/or structure(Reference Desai, Gayle and Babu32–Reference Desai, Crowther and Lucas35). However, in the present study, the rapeseed oil used seems to be more effective at maintaining brain, liver and testis fractional mass.
Protein restriction during gestation or lactation programmes the lipid metabolism in the offspring(Reference Lucas, Baker and Desai36), and the ingestion of control diet after weaning until 110 d was associated with higher cholesterol and TAG, in males, but not in females(Reference Zambrano, Bautista and Deás37). Nevertheless, the present results showed that post-weaning treatment with high-fat diets, enriched in vegetable oils (soya or rapeseed), after neonatal malnutrition seems to increase HDL-cholesterol, and it can improve the lipid profile. In the normal animals, this improvement seems to be better, since they showed lower TAG and higher HDL-cholesterol, after 60 d of vegetable oil diet. However, other authors have shown that treatment with soya or olive oils for two generations is associated in the second generation with higher cholesterol and TAG and lower HDL-cholesterol(Reference Fernandez, Gonzalez and Diaz38).
The relationship between thyroid hormones and metabolism has been studied in experimental models of protein or energy restriction during lactation. It was reported that in both cases serum thyroid hormones were higher in the adult animal(Reference Passos, Ramos and Dutra7). In the present model, the post-weaning recovery with high-fat diets suggests that those programming effects are changed in young rats. Instead of hyperthyroidism, the treatment with 19 % soya oil to the FR group programmed for low thyroxinaemia. In spite of this, it seems that this effect did not affect the other data in the present study, especially the total cholesterol. To our best knowledge, there is no report of the effects of vegetable oils upon thyroid function.
Hence, the interaction with neonatal malnutrition and the post-weaning recovery with higher-vegetable oil diets seem to ameliorate some features of the metabolic syndrome, such as the visceral fat mass and HDL-cholesterol, and change the programming effect of neonatal malnutrition upon thyroid function.
Acknowledgements
The authors thank Dr G. T. Boaventura and Dr E. Bouskela for the valuable assistance, Dr P. C. Lisboa for useful discussions and Mr Carlos Roberto for animal care. We affirm that there is no conflict of interest. Research was supported by the National Council for Scientific and Technological Development (CNPq), the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ) and Coordination for the Enhancement of Higher Education Personnel (CAPES). All authors have contributed to the work and are responsible for the content of the paper. C. A. S. C. treated the animals during pregnancy and lactation, manufactured the diets and measured the hormonal serum concentration; E. G. A. and G. P. L. G. controlled food intake, body and length development; V. D. L. and R. N. collected organs, weight and determined fat mass; A. S. C. determined serum concentrations of albumin, TAG, cholesterol and HDL-cholesterol; E. G. M. and C. C. A. N.-S. analysed the results and wrote the final version of the manuscript. All authors wrote some part of the manuscript and after reading the final version agreed to submit the paper to the British Journal of Nutrition.











