Endometrial cancer is the sixth most common form of cancer in women worldwide( Reference Ferlay, Ervik and Dikshit 1 ). It is strongly related to unopposed oestrogens, including both those produced endogenously in adipose tissue and those in the form of hormone replacement therapy (HRT). Other risk factors for endometrial cancer are also hormone related and include earlier menarche, nulliparity, overweight/obesity and older age at menopause, whereas oral contraceptive (OC) use is inversely related to endometrial cancer risk( Reference Purdie and Green 2 – Reference Keum, Greenwood and Lee 4 ).
Acute inflammation is the body’s natural response to insult or injury to tissue, which helps to heal wounds and promote tissue regeneration( Reference Keibel, Singh and Sharma 5 – Reference Warnberg, Gomez-Martinez and Romeo 8 ), whereas chronic inflammation is a persistent state of low-grade inflammation( Reference Warnberg, Gomez-Martinez and Romeo 8 ). Various dietary components are known to modulate inflammation( Reference de Mello, Schwab and Kolehmainen 9 – Reference Luciano, Mottus and Starr 11 ). Chronic inflammation is one of the major risk factors for development of several types of cancer( Reference Keibel, Singh and Sharma 5 , Reference Coussens and Werb 12 ), and considerable evidence has accumulated on the role of chronic inflammation in endometrial cancer( Reference Cust, Allen and Rinaldi 13 – Reference Friedenreich, Langley and Speidel 15 ). This more recent evidence is consistent with findings of Wynder et al.( Reference Wynder, Escher and Mantel 3 ) of the relationship between overweight and endometrial cancer from half a century ago. This line of evidence points towards a possible relation between inflammation deriving from dietary exposure and endometrial cancer risk.
A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet( Reference Shivappa, Steck and Hurley 16 ). A typical pro-inflammatory diet is characterised by high consumption of foods rich in SFA, carbohydrate and protein and low consumption of foods rich in PUFA, flavonoids and other selected components and micronutrients. The DII has been validated with various inflammatory markers, including C-reactive protein (CRP)( Reference Shivappa, Steck and Hurley 17 , Reference Wirth, Burch and Shivappa 18 ), IL-6( Reference Shivappa, Hebert and Rietzschel 19 , Reference Wood, Shivappa and Berthon 20 ) and homocysteine( Reference Ruiz-Canela, Zazpe and Shivappa 21 ). The DII has been shown to be associated with possible chronic inflammation-related conditions including intolerance and dyslipidaemia components of the metabolic syndrome in two cross-sectional studies in the USA and Luxembourg( Reference Ruiz-Canela, Zazpe and Shivappa 21 , Reference Alkerwi, Shivappa and Crichton 22 ), anthropometric measurements of obesity in the PREDIMED trial in Spain( Reference Ruiz-Canela, Zazpe and Shivappa 21 ), asthma in a case–control study in Australia( Reference Wood, Shivappa and Berthon 23 ), bone mineral density among postmenopausal women in Iran( Reference Shivappa, Hebert and Karamati 24 ), colorectal cancer in two cancer case–control studies, in Spain( Reference Zamora-Ros, Shivappa and Steck 25 ) and Italy( Reference Shivappa, Zucchetto and Montella 26 ), and in three cohort studies in women in the USA( Reference Shivappa, Prizment and Blair 27 – Reference Wirth, Shivappa and Steck 29 ) and pancreatic and prostate cancers in two Italian case–control studies( Reference Shivappa, Bosetti and Zucchetto 30 , Reference Shivappa, Bosetti and Zucchetto 31 ).
This study examined the relation between the DII and endometrial cancer risk in an Italian case–control study( Reference Bravi, Scotti and Bosetti 32 ), in order to test the hypothesis that a pro-inflammatory diet, as indicated by higher DII values, is associated with an increased risk for developing endometrial cancer.
Methods
The study was conducted between 1992 and 2006 in three Italian areas, including the greater Milan area, the provinces of Udine and Pordenone in northern Italy and the urban area of Naples in southern Italy( Reference Bravi, Scotti and Bosetti 32 ). Cases comprised 454 women (median age, 60 years; range, 18–79 years) with incident, histologically confirmed endometrial cancer (International Classification of Diseases, ninth edition, 182·0), admitted to major teaching and general hospitals of the study areas. Controls comprised 908 women (median age, 61 years; range, 19–80 years) admitted to the same hospital network as cases for a wide spectrum of acute non-neoplastic conditions. Women admitted for gynaecological or hormone-related conditions or for any medical condition related to long-term dietary changes were excluded. Controls were matched with cases by 5-year age groups and study centre, with a case:control ratio of 1:2. Of controls, 36 % were admitted for traumas, 32 % for other orthopaedic disorders, 9 % for acute surgical conditions and 23 % for other illnesses, including eye, nose, ear or skin disorders. Women with a history of hysterectomy were excluded from the control group. Less than 5 % of both cases and controls approached refused to be interviewed. All study participants signed an informed consent, according to the recommendations of the Institutional Review Boards of each study hospital.
Trained interviewers collected information on socio-demographic characteristics, anthropometric characteristics, lifestyle habits – including tobacco smoking and alcohol drinking – personal medical history and family history of gynaecological cancer, menstrual and reproductive factors, and OC and HRT use during their hospital stay using a structured questionnaire. There were few missing data (<1 %, as seen in Table 1); for these, we imputed values based on the most frequent category according to the subgroup to which they belonged. Subjects’ usual diet 2 years before cancer diagnosis or hospital admission (for controls) was assessed using an interviewer-administered FFQ, consisting of seventy-eight items on foods, including the most common Italian recipes, and five items on alcoholic beverages. Subjects were asked to indicate the average weekly frequency of consumption of each dietary item; intakes <1/week, but at least once a month, were coded as 0·5/week. Nutrient and total energy intake was determined using an Italian Food Composition Database. The FFQ showed reproducibility( Reference Franceschi, Negri and Salvini 33 , Reference Franceschi, Barbone and Negri 34 ) and satisfactory validity( Reference Decarli, Franceschi and Ferraroni 35 ) with Spearman’s correlation coefficients between 0·60 and 0·80 for most food items and nutrients.
Table 1 Distribution of 454 endometrial cancer cases and 908 controls according to selected variables (Italy, 1992–2006) (Number of cases and controls and percentages)

* The sum does not add up to the total because of some missing.
Dietary information obtained from the FFQ was used to calculate DII( Reference Shivappa, Steck and Hurley 16 ). Briefly, on the basis of a search of the literature from 1950 to the end of 2010, we identified forty-five food parameters among foods, nutrients and other food components that were associated with six plasma inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP). We defined a specific DII score for each food parameter on the basis of the literature review and by taking into account the quality and number of published papers (1943 articles were reviewed and scored).
For each study participant, the dietary data were first linked to a global database that was developed on the basis of eleven data sets from around the world and thus provides a robust estimate of the mean and the standard deviation of these forty-five parameters( Reference Shivappa, Steck and Hurley 16 ). Each subject’s exposure relative to the ‘standard global mean’ was expressed as a z-score that was derived by subtracting the ‘standard global mean’ from the amount reported and then dividing this value by its standard deviation. To minimise the effect of ‘right skewing’, this value was then converted to a centred percentile score. The subject’s DII score was computed by multiplying these values by the specific DII score for each food parameter and then summing together all these forty-five values according to the following formula, DII=b 1×n 1+b 2×n 2+………..+b 45×n 45, where b i refers to the literature-derived inflammatory effect score for each of the evaluated food parameter and n i refers to the food parameter-specific centred percentile, which were derived from the dietary data, per each i from 1 to 45. A higher DII score indicates a more pro-inflammatory diet. The DII computed on this study’s FFQ includes data on thirty-one of the forty-five food parameters comprising the DII including carbohydrate, protein, fat, alcohol, fibre, cholesterol, SFA, MUFA, PUFA, n-3, n-6, niacin, thiamin, riboflavin, vitamin B6, Fe, Zn, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavanol, flavonol, flavonones, flavones, isoflavones, caffeine and tea. A flow chart of the DII methodology is shown in Fig. 1. Energy, which is one of the components of DII, was adjusted for in the analyses. The thirteen food parameters missing from this study are onions, garlic, trans-fat, saffron, turmeric, thyme/oregano, ginger, vitamin B12, Se, pepper, rosemary, eugenol and Mg. DII scores were analysed both by quartiles of exposure computed among controls and as a continuous variable of an increment corresponding to approximately 10 % of its range (5·49 to −4·67).
OR and the corresponding 95 % CI were estimated using conditional logistic regression models conditioned on study centre and quinquennia of age and adjusted for year of interview, years of education (<7, 7–11, ≥12 years, categorically), BMI (categorically, by quartiles), age at menarche (<12, 12–13, ≥14 years, categorically), menopausal status/age at menopause (pre/perimenopause, <50, 50–54, ≥55 years, categorically), parity (0, 1, 2, 3, >3, categorically), history of diabetes (yes, no), family history of gynaecological cancers, OC use (never, ever) and HRT use (never, ever). Energy adjustment was made using the residual method, including also the term for total energy intake in the model( Reference Willett and Stampfer 36 ). Stratified analyses were carried out according to age (<55, 55–69, ≥70 years), BMI (<25, ≥25 kg/m2), menopausal status (pre/peri and postmenopause), parity (0/≥1 birth), OC use (yes, no) and HRT use (yes, no). To test for heterogeneity across strata, interaction terms were used. Linear tests for trend were performed using the median value within each quartile as an ordinal variable. Statistical tests were performed using SAS® 9.3 (SAS Institute Inc.).

Fig. 1 Sequence of steps in creating the dietary inflammatory index (DII) in the Italian endometrial case–control study. CRP, C-reactive protein.
Results
The distribution of 454 endometrial cancer cases and 908 controls according to age, education and other selected variables is presented in Table 1. By design, cases and controls had the same age distribution. As compared with controls, cases had a higher BMI and a lower age at menarche, reported more frequently a history of diabetes, and were less frequently OC users, multiparous and more frequently HRT users. Cases and controls were comparable in terms of education and menopausal status.
The mean energy-adjusted DII value for this study was 1·16 (sd 1·45). Cases had a higher mean DII value (0·05 (sd 1·40)) compared with controls (−0·03 (sd 1·48)). Characteristics of control subjects across quartiles of DII are provided in Table 2. There were small, non-significant differences in socio-demographic characteristics, anthropometric measures and hormone-related factors across quartiles of DII.
Table 2 Participants’ characteristics across energy-adjusted quartiles of dietary inflammatory index (DII) among 908 controls (Italy, 1992–2006) (Numbers and percentages)

* χ 2 Test for categorical variables.
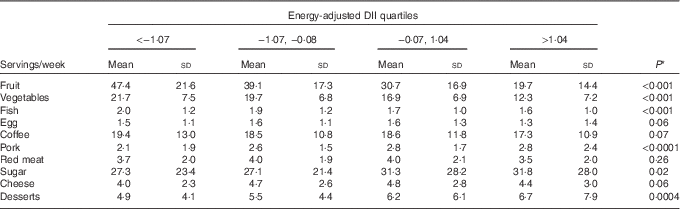
Table 3 shows the distribution of ten food groups across energy-adjusted DII quartiles among controls. Servings of fruit, vegetables and fish decreased significantly across quartiles, whereas servings of pork, sugar and desserts increased significantly.
Table 3 Distribution of servings of food groups across energy-adjusted quartiles of dietary inflammatory index (DII) among 908 controls (Italy, 1992–2006) (Mean values and standard deviations)
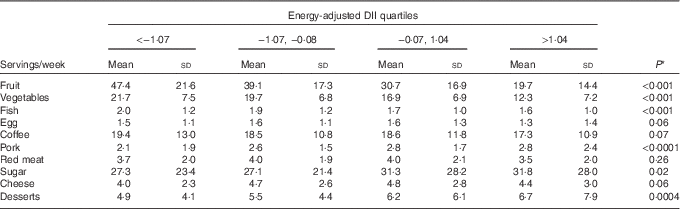
* This is the P value for the test of trend across DII quartiles.
Table 4 shows the OR of endometrial cancer for the highest v. the lowest energy-adjusted DII quartile and adjusted for all selected covariates. Significant positive associations were found: women in the fourth quartile of DII had an increased OR for endometrial cancer compared with women in the lowest quartile (ORQuartile 4 v. 1 1·46; 95 % CI 1·02, 2·11; P trend=0·04). A nearly significant positive association was observed when considering the DII as continuous OR for one unit increment of the DII (corresponding to approximately 10 % of its range) (OR 1·07; 95 % CI 0·98, 1·17; P=0·1), suggesting caution of interpretation. When we further adjusted for tobacco smoking and alcohol, the OR did not substantially change.
Table 4 Odds ratios of endometrial cancer for energy-adjusted quartiles of dietary inflammatory index (DII) among 454 cases and 908 controls (Italy, 1992–2006) (Odds ratios and 95 % confidence intervals)

* Conditioned on study centre and quinquennia of age and adjusted for energy.
† Reference category.
‡ Model 1 additionally adjusted for year of interview, education, BMI, age at menarche, menopausal status and age at menopause, parity, history of diabetes, family history of gynaecological cancers, oral contraceptive use and hormone replacement therapy use.
In Table 5, results are presented according to strata of selected covariates. Apparently, stronger associations were observed between DII and endometrial cancer among postmenopausal women (ORQuartile 4 v. 1 1·57; CI 1·04, 2·38), parous women (ORQuartile 4 v. 1 1·57; CI 1·05, 2·34) and non-HRT users (ORQuartile 4 v. 1 1·63; CI 1·10, 2·39). These results should be viewed with caution because of the absence of significant heterogeneity. The P value for heterogeneity was 0·44 for age, 0·34 for BMI, 0·16 for menopausal status, 0·78 for parity and 0·70 for HRT.
Table 5 Odds ratios of endometrial cancer according to energy-adjusted quartiles of dietary inflammatory index (DII) among 454 cases and 908 controls in strata of selected covariates (Italy, 1992−2006) (Odds ratios and 95 % confidence intervals)

HRT, hormone replacement therapy.
* Estimated from multiple logistic regression models, conditioned on study centre and quinquennia of age, and adjusted for year of interview, education, BMI, age at menarche, menopausal status and age at menopause, parity, history of diabetes, family history of gynaecological cancers, oral contraceptive use, HRT use and total energy intake.
† Reference category.
Discussion
The present study, one of the largest case–control investigations on diet and endometrial cancer to date in a southern European population, shows positive associations between DII and endometrial cancer. This result supports the hypothesis that women with a pro-inflammatory diet have a higher risk for developing endometrial cancer.
Previous results from this study indicate foods such as vegetables and coffee as well as various compounds including proanthocyanidins and β-carotene to be inversely related to endometrial cancer risk( Reference Bravi, Scotti and Bosetti 32 , Reference Rossi, Edefonti and Parpinel 37 – Reference Galeone, Augustin and Filomeno 43 ). All of these dietary factors contribute to lower DII values( Reference Shivappa, Steck and Hurley 16 ). Conversely, dietary patterns rich in animal products, starch and SFA, which contribute to higher DII values( Reference Shivappa, Steck and Hurley 16 ), were positively related to endometrial cancer risk( Reference Bravi, Bertuccio and Turati 44 ). An anti-inflammatory diet – as reflected in lower DII scores – also contains foods that are rich in antioxidant vitamins, flavonoids and fibre. These nutrient-dense, low-energy-content components combine to help keep the BMI low( Reference Rossi, Negri and Bosetti 45 ). Thus, it may not be possible to disentangle these different aspects.
The positive relationship between DII and endometrial cancer is consistent with a body of evidence from studies examining the effect of various dietary components on endometrial cancer. These include observational studies indicating a modest positive association between high glycaemic load, but not glycaemic index, and endometrial cancer( Reference Nagle, Olsen and Ibiebele 46 , Reference Turati, Galeone and Gandini 47 ). A meta-analysis of case–control studies suggests that meat consumption, particularly red meat, increases endometrial cancer risk, whereas poultry, fish and eggs produce inconsistent associations( Reference Bandera, Kushi and Moore 48 ). A protective role was observed for coffee consumption in endometrial cancer risk in the NIH-AARP (National Institutes for Health-American Association of Retired Persons) study( Reference Gunter, Schaub and Xue 49 ) and in the PLCO (Prostate, Lung, Colorectal and Ovarian Colorectal Cancer Screening Trial) cohort( Reference Hashibe, Galeone and Buys 50 ). A systematic review showed inverse associations between non-preserved vegetable intake and endometrial cancer, but a direct association with preserved vegetable intake( Reference Gallicchio, Matanoski and Tao 51 ). The latest report from World Cancer Research Foundation on diet and endometrial cancer indicated a probable protective role of coffee and increased risk from glycaemic load( 52 ). Additionally, in a simulated study, macrobiotic and Mediterranean meal plans exhibited anti-inflammatory potential, based on the derived DII scores, whereas a fast food diet had a pro-inflammatory score( Reference Steck, Shivappa and Tabung 53 ).
Previous studies have shown inflammation to be associated with endometrial cancer. Results from the Women’s Health Initiative showed that CRP was positively associated with endometrial cancer( Reference Wang, Rohan and Gunter 54 ). In a Canadian case–control study, endometrial cancer cases had consistently higher mean levels of TNF-α, IL-6 and CRP compared with controls in predominantly postmenopausal women( Reference Friedenreich, Langley and Speidel 15 ). In a case–control study, nested within the European Prospective Investigation into Cancer and Nutrition, higher levels of pro-inflammatory cytokines (CRP, IL-6 and IL-1Ra) were associated with increased risk for endometrial cancer( Reference Dossus, Rinaldi and Becker 14 ).
One of the possible mechanisms responsible for the observed positive association between the DII and endometrial cancer could be through the effect of pro-inflammatory diet on insulin resistance via increasing systemic inflammation( Reference Esmaillzadeh, Kimiagar and Mehrabi 55 , Reference Festa, D’Agostino and Howard 56 ). Consumption of foods such as meat and butter have been shown to increase systemic inflammation by increasing levels of high-sensitivity CRP, E-selection and soluble vascular cell adhesion molecule-1( Reference Esmaillzadeh, Kimiagar and Mehrabi 55 ), which then are responsible for increasing insulin resistance( Reference Festa, D’Agostino and Howard 56 , Reference Kaaks, Lukanova and Kurzer 57 ). Increasing insulin resistance then leads to increased circulating levels of insulin that has been demonstrated to play a role in the development of endometrial cancer by inhibiting apoptosis and stimulating cell proliferation( Reference Cust, Allen and Rinaldi 13 ) and by influencing the insulin-like growth factor axis, resulting in alterations in sex hormone metabolism( Reference Kaaks, Lukanova and Kurzer 57 ). The association was apparently stronger in postmenopausal women, as for several reproductive and hormonal correlates of endometrial cancer( Reference La Vecchia, Franceschi and Decarli 58 ). These results indicate a possible aetiological role of diet-associated inflammation, as indicated by the DII, in the development of endometrial cancer in scenarios where hormonal exposures are generally low.
With reference to possible sources of bias, dietary habits of hospital controls may differ from those of the general population( Reference Breslow and Day 59 ). In this study, however, we excluded from the control group all diagnoses that might have involved any obvious connection to dietary intake or to health-related changes in diet. With reference to information bias, cases and controls were interviewed in similar settings, and data provided by hospital controls have shown satisfactory reproducibility( Reference D’Avanzo, La Vecchia and Katsouyanni 60 ). The nearly complete participation of cases and controls and the inclusion of acute conditions unrelated to diet in the comparison group militate against a major role of selection bias. Furthermore, awareness of dietary hypotheses in endometrial cancer aetiology was unlikely in this Italian population. Another limitation is the non-availability of the remaining thirteen food parameters for the DII calculation. DII scores calculated from these thirty-one food parameters have not been validated with inflammatory markers. However, in previous validation studies, DII scores have been calculated from food parameters ranging from seventeen to forty-four( Reference Shivappa, Steck and Hurley 17 , Reference Shivappa, Hebert and Rietzschel 19 ). Moreover, there could be a possible overestimation due to the inclusion of food items in the DII calculation that are also a source of nutrients. It also should be noted, however, that each of these food items has an inflammatory effect score, which is derived from an extensive review of the literature looking at the association between these foods and inflammation. Among the strengths of our study were the large data set, the similar catchment areas across cases and controls and the satisfactory validity of information collected on dietary habits( Reference Franceschi, Negri and Salvini 33 , Reference Decarli, Franceschi and Ferraroni 35 ). Also, the DII score, which takes into account both pro- and anti-inflammatory food parameters that characterise virtually all human diets, more accurately reflects the relationship of the inflammatory potential of diet to affect cancer risk than would nutrients considered individually.
In conclusion, this unique, large study on endometrial cancer and DII indicates a possible role of diet in endometrial cancer risk through the process of inflammation. Confirmatory results from other studies are required to establish this association.
Acknowledgements
This study was supported by the Italian Foundation for Research on Cancer and by the Italian Ministry of Health, General Directorate of European and International Relations. N. S. and J. R. H. were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. N. S. is an employee of CHI. The subject matter of this paper has not had any direct bearing on that work, nor has that activity exerted any direct influence on this project.
The authors’ contributions were as follows: A.Z., M.M., C.L.V. and D.S. designed and conducted the case-control study, M.R. created the dataset for analyses, N.S. calculated DII and conducted all analyses and wrote the first draft of the manuscript, J.R.H., C.L.V., M.M., C.L.V., A.Z. and M.R. provided suggestions and revised the manuscript. All authors approved the final version of the manuscript.
There are no conflicts of interest.