INTRODUCTION
The development of targeted molecular therapies for cancer treatment in recent years has significantly decreased the risk of neutropenia in this group of patients [Reference Crawford, Dale and Lyman1, Reference Tamura and Fukuoka2] and new chemotherapeutic approaches for patients with solid tumours have substantially decreased neutropenia-related toxicity. However, most cancer patients still receive conventional chemotherapy as part of their treatment regimen. The majority of patients with haematological malignancy are at high risk of neutropenic infection post-chemotherapy and febrile neutropenia remains a major complication [Reference Sharma and Lokeshwar3, Reference Maschmeyer4]. Febrile neutropenia is associated with high morbidity and mortality in these patients and the overall prognosis is dependent on timely and adequate empirical antibiotic therapy [Reference Crawford, Dale and Lyman1, Reference Kuderer5].
Although several guidelines for the management of febrile neutropenia in cancer patients have been developed [Reference Hughes6–Reference Link8], the epidemiology of microbial pathogens and antimicrobial resistance may differ by geographical region [Reference Rolston9–Reference Ramphal11], and thus impact on their universal applicability. Studies from the USA and Europe show that Gram-positive microorganisms are the predominant isolates from blood cultures [Reference Wisplinghoff12, Reference Viscoli13], but the spectrum of bacteraemic pathogens in patients of febrile neutropenia with haematological malignancy in Taiwan, where Gram-negative organisms predominate, is clearly different from that in Western countries [Reference Chen10, Reference Wang, Lin and Liu14]. In addition, Taiwan has witnessed the emergence of fluoroquinolone-resistant Escherichia coli and Klebsiella pneumonia between 1996 and 2001 [Reference Chen10]. Antimicrobial resistance has serious consequences in neutropenic cancer patients contributing to high rates of treatment failure, prolonged infections, morbidity and mortality. Indeed, the emergence of fluoroquinolone-resistant E. coli and K. pneumoniae, carbapenem-resistant Acinetobacter spp., Stenotrophomonas maltophilia as well as vancomycin-resistant Enterococcus faecium have all been reported in neutropenic patients [Reference Chen10, Reference Safdar and Rolston15–Reference Cattaneo18]. It follows that regular monitoring of the epidemiology of bacterial infections allows evaluation of antibacterial strategies and their adaptation to lessen the impact of emerging pathogens [Reference Mutnick, Kirby and Jones19].
In this study, we examined the spectrum of bloodstream isolates from patients with haematological malignancies attending a medical centre in Taiwan between 2002 and 2006 and correlated the pathogens recovered with clinical characteristics and antimicrobial resistance.
PATIENTS AND METHODS
Setting and patients
National Taiwan University Hospital (NTUH) is a 2000-bed teaching hospital in metropolitan Taipei that provides both primary and tertiary care. The medical records of patients admitted to the haemato-oncology wards of the hospital between 1 January 2002 and 31 December 2006 were reviewed. All patients with haematological malignancies were enrolled into the study, including acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL), non-Hodgkin's lymphoma, multiple myeloma (MM), chronic myeloid leukaemia (CML), chronic lymphocytic leukaemia (CLL), myelodysplastic syndrome (MDS), aplastic anaemia (AA), and others. Patients with solid cancers and non-cancer patients were excluded. Most patients were admitted to receive induction or consolidation chemotherapy or were undergoing haematopoietic stem cell transplantation. Other patients were admitted for the treatment of complications of malignancy such as infectious disease or bleeding.
A complete physical examination was performed at baseline and at least once daily during therapy for malignancy. Imaging by computed tomography scan, ultrasound and other examinations were performed if indicated according to clinical conditions. Before the start of antibiotic therapy, full blood count, liver biochemistry, renal function test, and chest X-ray, were undertaken. Two or three sets of blood cultures (aerobic and anaerobic bottles) with at least one from a peripheral vein were set up using the Bactec 9240 system (Becton Dickinson, USA). Urinalysis and urine culture were also performed. Bacteria and fungi were isolated by conventional methods and, if necessary, species identity was confirmed by the Phoenix identification systems (Becton Dickinson). Routine anti-bacterial or anti-fungal prophylaxis was not prescribed for patients receiving chemotherapy at NTUH during the study period.
Definitions
Febrile neutropenia was defined according to the criteria of the Infectious Disease Society of America [Reference Hughes6]. Fever was defined as an axillary temperature of ⩾38·3°C on one occasion or of >38·0°C on two or more occasions during a 12-h period. Neutropenia was a neutrophil count <500 cells/mm3 or a count <1000 cells/mm3 with a predicted decrease to <500 cells/mm3. Infections were classified as community acquired if fever developed within 72 h of admission, while development of fever after this time indicated nosocomial infection.
Antimicrobial susceptibility
Data on the susceptibilities of bloodstream isolates determined by the disk diffusion method [20] during the study period were retrieved from the annual summary document. Non-duplicate isolates of each species with identical resistance profiles recovered from each patient within 7 days were noted to calculate resistance rates. Detection of extended-spectrum β-lactamase (ESBL) phenotypes in E. coli, K. pneumoniae, Proteus mirabilis, and K. oxytoca isolates began in 2003 using methods in accordance with the Clinical and Laboratory Standards Institute (CLSI) [20, 21]. Isolates with intermediate resistance or fully resistant to antimicrobial agents were classified as a resistant phenotype. Extensively drug-resistant Acinetobacter calcoaceticus-baumannii complex (XDRAB), isolates were defined as being resistant to all agents tested, including ampicillin-sulbactam, ceftazidime, cefepime, piperacillin-tazobactam, aztreonam, imipenem, meropenem, ciprofloxacin, levofloxacin, gentamicin, and amikacin, with the exception of colistin. A broth microdilution method was used for susceptibility testing of colistin and classified according to CLSI guidelines (susceptible ⩽2 μg/ml, resistant ⩾4 μg/ml) [21].
Antimicrobial prophylaxis and treatment
Prophylactic use of an oral fluoroquinolone (levofloxacin or moxifloxacin) was recommended in high-risk populations (e.g. acute leukaemia and bone marrow transplantation) in whom chemotherapy-induced neutropenia (<500 neutrophils/mm3) was expected to last >7 days. Antifungal prophylaxis was not routinely used after chemotherapy. Empirical antibiotic treatment for patients with febrile neutropenia followed published guidelines [Reference Hughes22, 23].
Statistics
Statistical comparisons were made with χ2 test using SPSS for Windows, version 13.0 (SPSS Inc., USA). A P value of <0·05 was considered to indicate a significant difference.
RESULTS
Underlying haematological diagnosis
There were 7058 admissions to the haemato-oncology wards during the 5-year study period, including 3974 (56%) patients with haematological malignancies and 1032 (15%) patients with solid cancers. Seventy-eight percent of all admissions were patients with acute leukaemia (28% AML, 9% ALL) and lymphoma (41%).
Microbial aetiology
A total of 1307 non-duplicate isolates was recovered from blood cultures in patients with haematological malignancy admitted during the study period; 853 (65%) were isolated from patients with neutropenia. Figure 1 shows that over the study period Gram-negative infections in neutropenic patients peaked in 2005 at 65% but fell in 2006 to frequencies similar to the start of the study. On the other hand infections with Gram-positive organisms ranged from 35% to 45% (P=0·255) and Candida spp. from 5% to 9%. There was a similar range and frequency of species recovered from all patients with and without neutropenia (Table 1). The leading Gram-negative pathogens were E. coli, K. pneumoniae, A. calcoaceticus-baumannii complex and S. maltophilia. The number of A. calcoaceticus-baumannii complex and S. maltophilia isolates increased over the study period and were more common than Enterobacter cloacae and Pseudomonas aeruginosa. Coagulase-negative staphylococci, including Staphylococcus epidermidis, were the most commonly isolated Gram-positive bacteria in both groups of patients; S. aureus comprised 4% and 5% of isolates from neutropenics and all patients, respectively, and 56% of these were oxacillin resistant and thus classified as MRSA. A similar patient distribution was observed for Streptococcus spp. with the majority being viridans streptococci (83% neutropenics, 90% all patients). Equal numbers of Enterococcus faecalis and E. faecium were isolated from neutropenic patients. Episodes of Candida bloodstream infections accounted for 6% and 5%, respectively, of neutropenic with all patients with C. tropicalis and C. albicans being the most frequent.
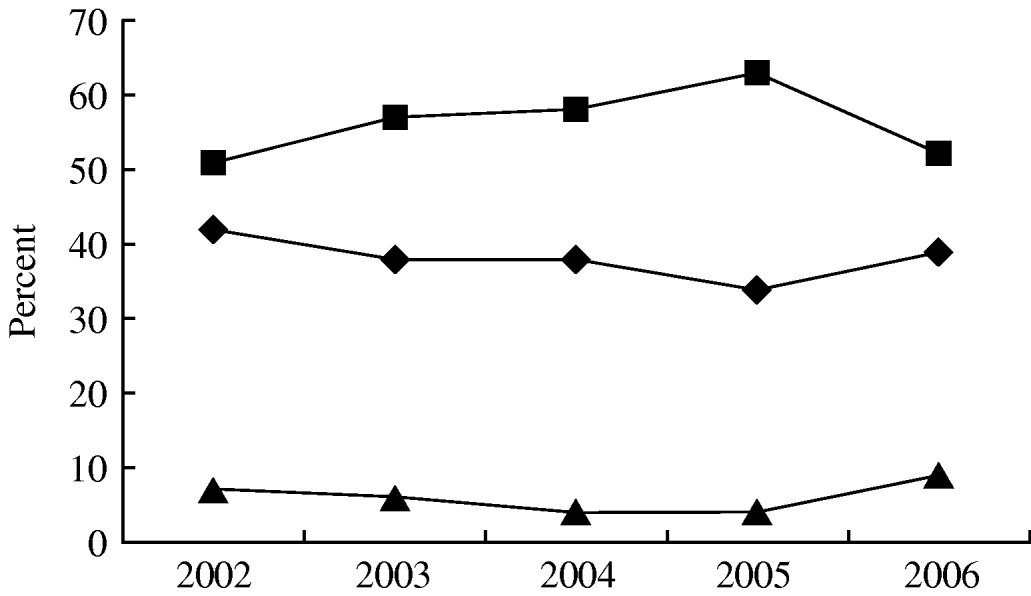
Fig. 1. Aetiological trends of bloodstream infections in febrile neutropenic adults with haematological malignancy at National Taiwan University Hospital from 2002 to 2006. χ2 trend analysis (P=0·255). –◆–, Gram-positive pathogens; –▪–, Gram-negative pathogens; –▴–, fungus.
Table 1. Pathogens isolated from blood in patients with haematological malignancy with and without neutropenia at National Taiwan University Hospital from 2002 to 2006
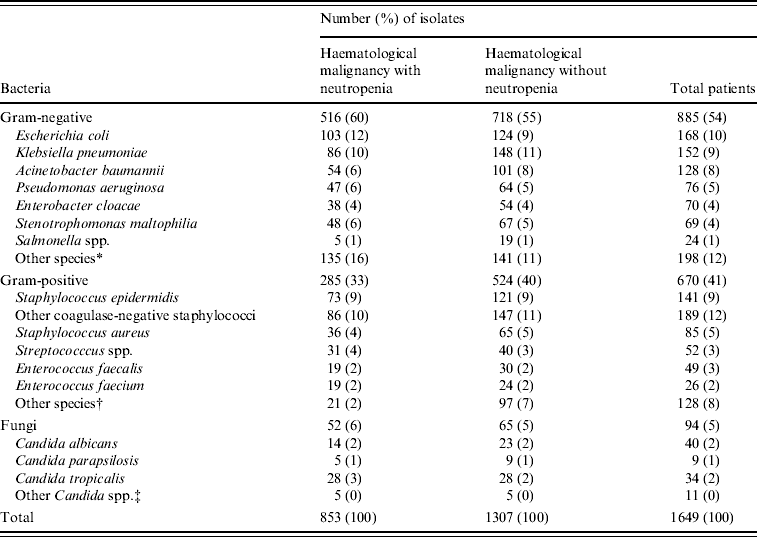
* Including Proteus mirabilis, Serratia marcescens, Acinetobacter Iwoffii, Aeromonas hydrophila, and Sphingomonas paucimobilis.
† Including Corynebacterium spp., Bacillus spp., and Micrococcus.
‡ Including Candida krusei, C. glabrata, and C. guilliermondii.
The distribution of microbial species in neutropenic patients according to type of haematological malignancy is shown in Table 2. Patients with AML accounted for over half (52%) of all pathogens recovered despite the fact that they represented just over a quarter (1093/3974, 27·5%) of all admissions. Similarly, patients with ALL represented 9·4% (375/3974) of those admitted during the study period with haematological malignancies but accounted for only 20% of all isolates from neutropenic patients. A similar picture was observed for non-Hodgkin's lymphoma patients, i.e. 41% of admissions, 17% of blood isolates from neutropenia.
Table 2. Distribution of bloodstream pathogens in patients according to haematological malignancies
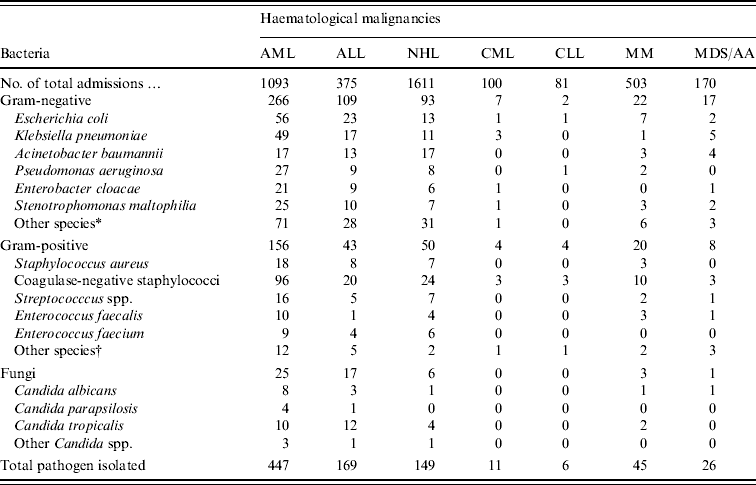
AA, Aplastic anaemia; AML, acute myeloid leukaemia; ALL, acute lymphoblastic leukaemia; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma.
* Other species of Gram-negative bacteria: Serratia marcescens, Acinetobacter Iwoffii, Aeromonas hydrophila, Proteus mirabilis, and Sphingomonas paucimobilis.
† Other species of Gram-positive bacteria: Corynebacterium spp., Bacillus spp., and Micrococcus spp.
Antibiotic susceptibility
There were no statistically significant differences between the patient groups with regard to antimicrobial susceptibility (Table 3). The only notable resistance was that 50% of E. coli and 20% of K. pneumoniae isolates from neutropenic patients were resistant to ciprofloxacin. Similar rates of resistance were also observed for those without neutropenia. Production of ESBLs was detected in 12% and 3%, respectively, of E. coli and K. pneumoniae from patients with neutropenia.
Table 3. Susceptibilities of isolates from patients with haematological malignancy with and without neutropenia during 2002–2006
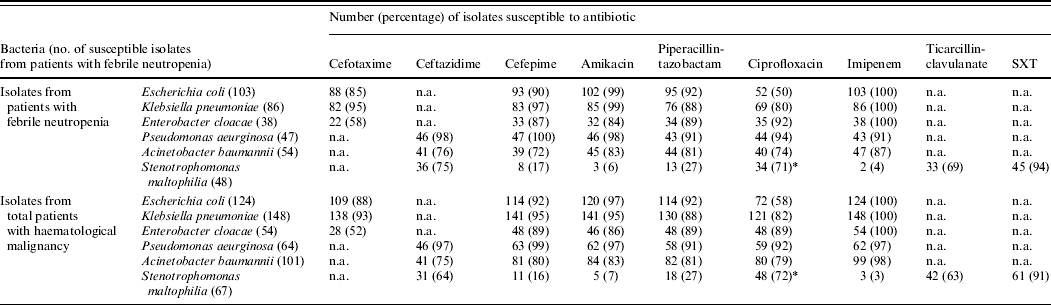
n.a., Not available; SXT, sulfamethoxazole–trimethoprim.
* For S. maltophilia isolates, levofloxacin was tested instead of ciprofloxacin.
Isolates of E. cloacae were on the whole susceptible to amikacin (84%), and piperacillin-tazobactam (89%) but exhibited variable susceptibility to cefotaxime; however, all isolates were susceptible to imipenem. P. aeruginosa from both groups of patients were invariably susceptible to all antipseudomonal agents while most of A. calcoaceticus-baumannii complex isolates from all patients were susceptible to imipenem, piperacillin-tazobactam and amikacin; however, 6% of isolates of this complex from neutropenic patients and 4% of the control group were found to be be multiresistant to all antimicrobials (XDRAB). S. maltophilia isolates were susceptible to sulfamethoxazole-trimethoprim (>90%) but less so to ceftazidime, and levofloxacin. Of the Gram-positive isolates oxacillin resistance was exhibited by over half (56%) of S. aureus from neutropenic patients and was marginally reduced for other patients (49%). Penicillin resistance in viridians streptococci was 14% in both patient groups and 21% of E. faecium isolates were vancomycin resistant.
Clinical characteristics and mortality
Most patients admitted to the haemato-oncology wards received chemotherapy and developed fever during neutropenic episodes; 5% of these were blood culture-positive within 72 h of admission. Although most patients required frequent admissions for chemotherapy or supportive care, only one isolate of A. calcoaceticus-baumannii complex and none of S. maltophilia were found in patients with community-acquired infection. C. tropicalis was the most common fungal infection in patients with febrile neutropenia, but only two patients with community-acquired infection grew C. albicans. Overall, there was no significant difference in microbiological spectrum between patients with community-acquired and nosocomial infection. There were 193 (23%) isolates from patients with one episode of febrile neutropenia and this rose to 660 (77%) from patients with two or more febrile episodes. K. pneumoniae was less common in the latter group.
A total of 102 (12%) of 853 pathogens was isolated within 14 days from patients who died. The median duration between isolate positive blood culture and death was 5 days. E. coli (6%) and S. maltophilia (35%) isolates were identified within 14 days before mortality. The 14-day outcome for neutropenic patients with E. coli was better than that for neutropenic patients with other microbial species (P=0·036). By contrast, the outcomes for neutropenic patients with S. maltophilia bloodstream infections were significantly worse than for other patients (P<0·001).
DISCUSSION
This retrospective study disclosed several important points. First, Gram-negative bacteria were the predominant pathogens (60%) and fungal infections were relatively uncommon (6%) in bloodstream infections in patients with neutropenia. Second, the number of Acinetobacter and Stenotrophomonas infections increased from 2002 to 2006 and were the third (7%) and fourth (6%) most frequent after E. coli and Klebsiella. Last, an increasing burden of antimicrobial resistance was noted in several pathogens; >40% quinolone resistance and 12% of ESBL producers in E. coli isolates, 6% of A. calcoaceticus-baumannii complex isolates exhibiting resistance to several agents and 21% of E. faecium with vancomycin resistance.
Several studies in recent years have reported a shift from Gram-negative infections towards Gram-positive infections in cancer patients with febrile neutropenia [Reference Cattaneo18, Reference Viscoli and Castagnola24–Reference Wisplinghoff27]. However, Gram-negative bacteria have predominated in such patients in most reported studies from Taiwan [Reference Chen10, Reference Wang, Lin and Liu14]. The reasons why Gram-negative pathogens have continued to be the most prevalent in NTUH in the last 10 years compared to the previous survey from 1996 to 2001 [Reference Chen10] remain unknown. These findings underline the need for regular surveillance of the epidemiology and antimicrobial resistance of pathogens in different geographic areas to determine appropriate empirical antibiotic treatment for neutropenic cancer patients.
A. calcoaceticus-baumannii complex and S. maltophilia in bloodstream isolates of neutropenic patients represent around 1–3% in the USA and Europe [Reference Wisplinghoff27, Reference Klastersky28]. However, in some areas the frequency of A. calcoaceticus-baumannii complex in neutropenic patients is reportedly higher at 6–9% [Reference Chen10, Reference Irfan16, Reference Velasco29] and several studies have noted an increasing frequency of S. maltophilia infection in these patients [Reference Safdar and Rolston15, Reference Ansari17, Reference Aisenberg30].
We found no significant difference in the microbiological spectrum between neutropenic patients with community-acquired and nosocomial infection and this might be explained by the fact that these patients have frequent admissions for chemotherapy or supportive care and thus the distinction between community-acquired and nosocomial infection is less rigid.
The emergence of quinolone-resistant E. coli in neutropenic cancer patients has been observed in several institutions in Europe since 1994 [Reference Cattaneo18, Reference Cometta31] and in our hospital increased from 33% to 50% between 1996–2001 and 2002–2006. Ciprofloxacin-resistant K. pneumoniae isolation also increased (13–20%) over the two periods. This may reflect the more widespread use of quinolones for prophylaxis in high-risk populations. Nevertheless, during this study period, the susceptibility of E. cloacae to cefotaxime, amikacin, and piperacillin-tazobactam increased from 38% to 58%, 71% to 84%, and 75% to 89%, respectively. Ceftazidime and carbapenems remained effective agents for P. aeruginosa infection. There was a decrease in the activity of antibiotics against A. baumanii isolates over the two periods (83–94% susceptible in 1996–2001 and 72–87% in 2002–2006). Carbapenem resistance in this complex has been reported to have risen in the USA from 9% in 1995 to 40% in 2004 [Reference Munoz-Price and Weinstein32]. We found no extreme resistance in this group in the first survey [Reference Chen10] unlike the current study. Multiresistant Acinetobacter infections tend to occur in immunocompromised patients, those with serious underlying diseases, and in those subjected to invasive procedures and/or treated with broad-spectrum antibiotics [Reference García-Garmendia33, Reference Perez34].
Patients with prolonged neutropenia, exposure to broad-spectrum antibiotics such as the carbapenems, and those requiring mechanical ventilation are at increased risk of S. maltophilia infection [Reference Safdar and Rolston15]. Our neutropenic patients with positive blood cultures of S. maltophilia had significantly worse 14-day outcome than other groups and 76% of S. maltophilia isolates were recovered from neutropenic patients with refractory disease status. Further studies are necessary to clarify the relationship between S. maltophilia infection and poor outcome in patients with haematological malignancy taking into account other variables potentially associated with poor outcome.
In conclusion, several opportunist pathogens, some exhibiting significant antimicrobial resistance, are increasing as the causes of infection in neutropenic cancer patients. Regular monitoring of bacterial epidemiology and antimicrobial resistance in these patients are crucial and will help to inform the suitability of local policies for the use of antimicrobial agents and the choice of agents for empirical antibiotic therapy, and prophylaxis in high-risk patients.
DECLARATION OF INTEREST
None.








