INTRODUCTION
Hepatitis C virus (HCV) infection is a serious global public health issue with an estimated prevalence of 2·2% worldwide [1]. About 75% (between 50% and 85%) of people infected develop chronic infection and between 5% and 20% of those who are chronically infected will develop cirrhosis after about 20 years. An estimated 4% of those with cirrhosis progress to decompensated liver disease and 1·6% develop hepatocellular carcinoma (HCC) annually [1]. Very effective treatment is available with a sustained response in over 50% of cases, depending upon the infecting genotype [2].
In order to develop prevention and treatment services for HCV, accurate information is needed regarding the numbers infected, their duration of infection and risk factors for acquisition. In 2006, the European Parliament identified the harmonization of surveillance of viral hepatitis in the European Union (EU) as one of the priorities for the European Centre for Disease Prevention and Control (ECDC). The ECDC subsequently published the results of a survey and a literature review on viral hepatitis infection in the EU and neighbouring countries in order to inform policy-making on primary and secondary prevention of viral hepatitis [3, 4]. Both reports identified that prevalence data on HCV in the general population in Europe were scarce, being available for only a minority of countries. Ireland was identified as one of the countries not having data on HCV prevalence in the general population.
The main causes of HCV infection in Ireland are injecting drug use and receipt of HCV-contaminated blood or blood products in the past. Opiate use increased substantially in Dublin in the late 1970s and early 1980s and lower prices precipitated a second epidemic in the 1990s [Reference O'Gorman, Korf and Riper5, Reference Dean6]. Studies of injecting drug users (IDUs) in prisons and drug users attending methadone clinics, specialist addiction treatment centres and general practitioners (GPs) have estimated the HCV antibody prevalence in this population to be between 50% and 84% [Reference Smyth7–Reference Comiskey and Cox16]. A capture–recapture study estimated the number of opiate users in Ireland in 2006 to be 20 790 [Reference Kelly, Teljeur and Carvalho17]. Testing for bloodborne infections is usually offered to drug users when they first attend drug treatment services and is repeated at regular intervals. Therefore, trends in HCV diagnoses in drug users are likely to be affected by trends in the availability of drug treatment and harm reduction services. Most of the drug-treatment clinics in Ireland were established in the early 1990s.
HCV diagnoses in people who were infected through contaminated blood or blood products are also affected by the introduction of screening programmes. Many of those who had received blood-clotting factors as treatment for coagulation disorders were tested for HCV infection once reliable tests became available in the early 1990s. In early 1994, it was discovered that anti-D immune globulin contaminated with HCV had been administered to a number of women between 1977 and 1979 and also between 1991 and 1994. In 1994, a targeted lookback exercise was initiated and this was followed in 1995 by further screening programmes for people who had received blood or blood products prior to the introduction of routine HCV antibody (anti-HCV) screening of blood-product donors in October 1991. About 1700 people infected with HCV through blood or blood products were identified [18].
Testing for anti-HCV began in Ireland in 1989 and about 95% of confirmatory investigations, both serological and molecular, were performed in the National Virus Reference Laboratory (NVRL) over the following 15 years. The NVRL has a laboratory information management system (LIMS) which is specimen-based rather than person-based. There is no unique personal health identification number system in use in Ireland. Therefore, as many HCV-infected people had multiple investigations performed, it has not been possible to count the total number of people who tested positive or their year of first diagnosis through this system. Risk-factor information on many cases is also recorded in the LIMS system, but not in a standardized format.
The primary aim of this study was to convert the NVRL specimen-based data to person-based data and to estimate the number of people who have tested HCV positive, by year of diagnosis and risk group from 1989 to 2004. A further objective of this study was to estimate the current prevalence of chronic HCV infection in Ireland by combining information from the NVRL system with national HCV notification data from 2004 onwards, when HCV infection became statutorily notifiable by all laboratories and clinicians in Ireland [19].
METHODS
Serological testing for HCV usually involves an initial anti-HCV screening test (ELISA/EIA), followed by an immunoblot confirmatory antibody test (RIBA). Anti-HCV tests identify both current and past infections and, if positive, samples are subsequently collected for HCV RNA testing to detect viraemic patients. All positive or weakly positive anti-HCV tests in the NVRL LIMS system were included in the original dataset for the study. Initially, all RNA results, positive and negative, were included in the dataset to permit the subsequent linking of these to each individual's records.
The patient demographics accompanying a sample often vary between samples collected from the same patient. Therefore, to ensure that all samples in the NVRL LIMS were appropriately linked to a given patient, Microsoft Access was used to create a database linking samples to patient demographics. Potentially matching pairs of laboratory records were assigned a score based on the number and type of demographic variables found to be equivalent. The variables used were name or equivalent name (e.g. Joe or Joseph), initials, date of birth, hospital number, source of test sample and gender. Each potentially matching pair was then assessed and either accepted or rejected, with potential matches with very high ranking scores (~79%) accepted automatically. This group included those based on having the same hospital number and source of sample, or name and date of birth. A sample of the automated matches was also manually checked to ensure that erroneous matching had not occurred. Potential matches with weaker scores (e.g. same forename, surname and birth year, but day or month of birth different) were manually reviewed.
Subsequently, the individual patient and test result data were imported into a custom-designed Microsoft Access database which had functionality to allow test results, reason for testing, source of sample and risk factor information to be interpreted and mapped to standardized values. The date of diagnosis of HCV infection was derived from the specimen date of the first positive or indeterminate anti-HCV result. Individuals were considered to be confirmed as HCV positive if they ever had a positive RNA result or had a positive RIBA result when aged >2 years.
An additional variable was created to record each individual's overall HCV status based on all of their test results. Risk-factor information was collected from several data fields, and when multiple risk factors were reported, the following hierarchy was used to decide which was the most probable route of transmission: drug use (available information did not allow for this to be further specified as injecting drug use), received blood/blood products, contact with a case or an at-risk individual, vertical exposure, accidental exposure to blood/body fluids (including assault) and sexual exposure.
In order to estimate the current prevalence of chronic HCV infection in Ireland, the NVRL data (1989–2004) and national notification data (2004–2009) were combined, and then adjusted using epidemiological information on HCV from published studies. The year 2004 (when HCV first became notifiable) was included in both datasets so that comparisons could be made between the two sources of information, with NVRL data taken as the ‘gold standard’ for new identifications of infection, while notification data could include some previously diagnosed cases being re-tested for the first time since HCV became notifiable. National notification data were obtained from the Health Protection Surveillance Centre (HPSC) computerized infectious disease reporting system (live system accessed in May 2010). The data were adjusted using the following assumptions:
-
(1) NVRL identified 95% of HCV diagnoses in Ireland from 1989 to 2004 (personal communication: S. Dooley, Laboratory Manager, NVRL). Adjustment: NVRL data were adjusted upwards to account for diagnoses by other laboratories.
-
(2) The level of discrepancy between national HCV notifications in 2004 and the adjusted NVRL data for 2004 continued into 2005–2009 (national notifications were higher – see Results section for details). Adjustment: national notification data adjusted downwards.
-
(3) Seventy-five percent of HCV infections remain chronic [1]. Adjustment: combined data adjusted downwards.
-
(4) Thirteen percent of those with chronic infection have died [Reference McLeod20]. Adjustment: combined data adjusted downward.
-
(5) Many cases of HCV infection are undiagnosed, ranging from a conservative estimate of one in two, to two in three as used in Scotland [Reference Hutchinson21] and four in five as used in England [22]. Adjustment: combined data adjusted upwards for each of these estimates to provide a range of chronic HCV population prevalence estimates for Ireland.
RESULTS
Overall numbers testing HCV positive, 1989–2004
A total of 10 384 individuals were confirmed as HCV infected, either currently or previously, by the NVRL between 1989 and 2004 (Table 1). There was an increasing trend in the number of cases identified between 1989 and 2000, after which the numbers decreased (Fig. 1). Seventy-eight percent of confirmed positive individuals had RNA results. Of these, 82% (n=6642) had one or more positive RNA results and 75% (n=6087) had a positive RNA result when last tested.
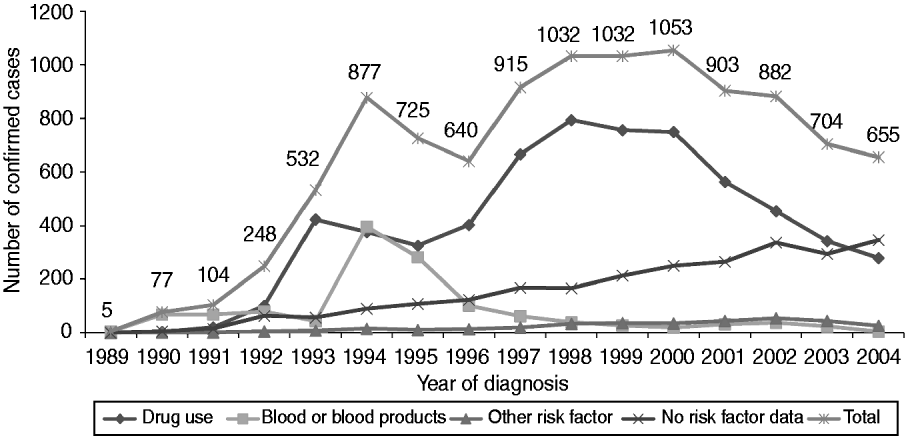
Fig. 1. Number of National Virus Reference Laboratory-confirmed cases by year of diagnosis and reported risk factor, 1989–2004.
Table 1. Number of individuals with positive HCV results, National Virus Reference Laboratory, 1989–2004
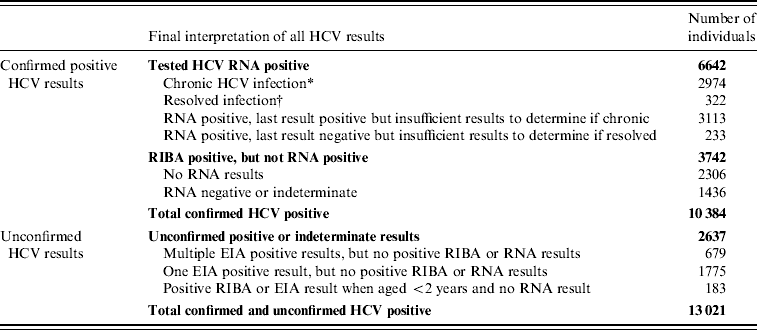
* Chronic infection=two or more positive RNA results ⩾6 months apart, with no negative RNA results in the interim or subsequently.
† Resolved infection=one or more positive RNA results, followed by two or more negative RNA results, ⩾6 months apart, with no positive results in the interim or subsequently.
It was not possible to determine the definitive HCV status of a further 2637 individuals.
Risk factors
It was possible to assign a most likely risk factor to 75·6% (n=7853) of all confirmed cases. Of these, the most likely risk factor was as follows: current or former drug use (n=6255, 79·7%), receipt of blood or blood products (n=1285, 16·4%), accidental exposure to blood/body fluids (including assault) (n=102, 1·3%), sexual exposure (n=98, 1·2%), vertical exposure (n=79, 1·0%) and contact with a case or an at-risk individual (n=34, 0·4%) (Fig. 1).
Age and sex
Age and sex were known for 98% of cases. Males predominated and represented 63% (n=6387) of all confirmed cases. The median age at diagnosis was 28 years. However, the age and sex distribution of cases varied significantly according to the mode of acquisition of infection.
Seventy per cent of drug users were male. With the exception of the early years of testing, when the numbers of cases diagnosed were low, the proportion of male cases in drug users each year was fairly consistent and ranged from 65% to 77%. The overall median age at diagnosis for those infected through drug use was 25 years for males and 23 years for females. This has increased slightly since 2001 and the median age for all identified drug use-related new diagnoses in 2004 was 28 years.
Cases infected through blood or blood products were older at diagnosis, with a median age of 34 years for males and 44 years for females. The sex distribution was also different to that of drug users with females accounting for 71% of cases. The predominance of females and their older age at diagnosis was due to the large cohort of females infected with hepatitis C through the administration of contaminated anti-D between 1977 and 1979 [18].
Genotype
Genotype was available for 61% of all confirmed positive individuals and for 95% of those who had positive HCV RNA results (n=6314). Of these, 55% (n=3493) were genotype 1, 39% (n=2444) were genotype 3, 4% (n=227) were genotype 2, 1% (n=62) were genotype 4 and 0·1% (n=6) were genotype 5. A small number of individuals had infection with more than one genotype, most commonly genotypes 1 and 3 (1·2%, n=73). However, with the exception of two individuals, different HCV genotypes were detected in separate samples and therefore probably reflect re-infections rather than co-infections. The genotype distribution differed between people infected through drug use and those infected through blood/blood products (Fig. 2). Seventy-one per cent of people infected through blood/blood products had genotype 1 infection, compared to 53% of drug users. Genotype 3 HCV was the second most prevalent in both, and accounted for 22% those infected through blood/blood products and 42% of drug users.
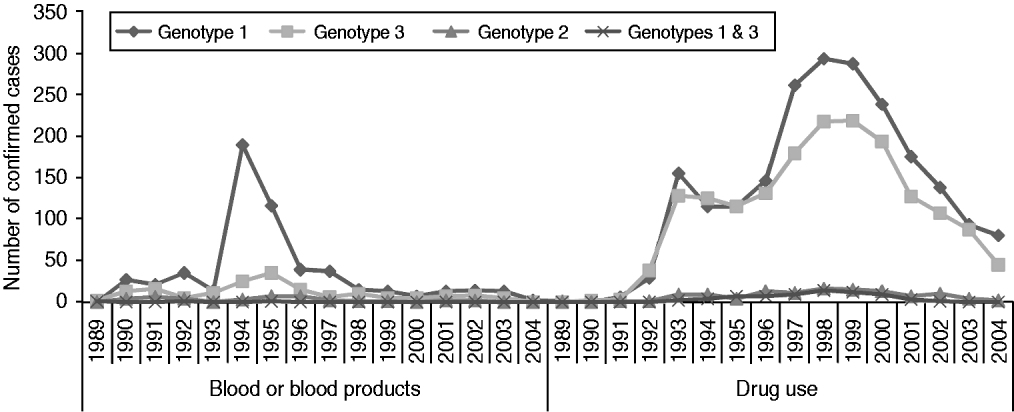
Fig. 2. HCV genotype by year of diagnosis, for those infected through blood or blood products or through drug use.
National HCV notification data and estimates of prevalence of chronic HCV in Ireland
A total of 8104 HCV cases were notified during 2004–2009 in a population of 4 239 848 (2006 census) [23]. Notifications peaked in 2007 (n=1554), declining to 1261 in 2009. There were 1128 notifications of HCV in 2004 compared to 655 new diagnoses by the NVRL in that year. The number of notifications remained substantially higher than the number of NVRL diagnoses after increasing the NVRL figure by 100/95 to account for testing by other laboratories. The most likely explanation for this discrepancy is that a significant portion of the cases notified in the early years after HCV became notifiable were diagnosed before 2004 but tested again as part of routine follow-up and then notified for the first time. It is also likely that there were some duplicate notifications. The NVRL data were taken as a better reflection of true number of new diagnoses. HCV notifications from 2005 to 2009 were adjusted downwards to account for the same level of over-reporting, resulting in a final estimate of 15 193 cases of HCV diagnosed in Ireland between 1989 and 2009. Essentially, the calculation was: (NVRL diagnoses 1989–2004×100/95)+(national notifications 2005–2009×61·1%). Assuming a chronicity rate of 75% and a mortality rate of 13% [18], we estimated that 9913 remain chronically infected.
Assumption 1:
NVRL identified 95% of HCV diagnoses in Ireland 1989–2004
Assumption 2:
The level of discrepancy between national HCV notifications in 2004 and the adjusted NVRL data for 2004 continued into 2005–2009
Assumption 3:
75% of HCV infections remain chronic
Assumption 4:
13% of those with chronic infection have died
As HCV is initially asymptomatic in about 75% of cases [1] the true number of individuals living with chronic HCV in Ireland is likely to be substantially higher than this. Assuming levels of under-diagnosis of 50%, 67% [Reference Hutchinson21] and 80% [22], this would indicate that the true number of chronically infected individuals who are alive ranges from 19 826 to 29 739 to 49 565. These equate to a population prevalence ranging from 0·5% to 0·7% to 1·2%.
DISCUSSION
This study provides the first comprehensive national estimate of the number of people infected with HCV in Ireland, along with trends in diagnosis, risk-factor information and genotype. This information on burden of disease is needed at both national and European levels to inform policy decisions on primary and secondary prevention. The novel approach used, whereby routinely collected specimen-based laboratory data were converted into useable information, is a method that could be replicated elsewhere.
By combining information from laboratory HCV diagnoses with national notification data and making adjustments, we estimate that over 15 000 individuals were diagnosed with HCV in Ireland by the end of 2009. Of these, almost 10 000 are likely to be still alive and remain chronically infected. After accounting for under-diagnosis, the population prevalence of chronic HCV is estimated to be between 0·5% and 1·2%, similar to other northern European countries. The prevalence of chronic infection is estimated to be 0·75% in Scotland [Reference Hutchinson21] and 0·4% in England [22]. The prevalence of anti-HCV in the general population in Sweden is reported to be about 0·5% [Reference Duberg24]. A recent systematic literature review of HCV prevalence in Europe carried out by the ECDC reported a prevalence range of 0·4–3·5% [4]. However, this was based on information from only 14 countries, and prevalence estimates that could be considered representative of the entire country were available for only three countries.
Over 10 000 individuals were confirmed as HCV infected by the national laboratory prior to 2005. Where risk-factor information was available, almost 80% were classified as drug users. Unfortunately, available information did not allow for further specification into injecting or non-injecting drug use. This increased to 90% when only cases diagnosed after 1996 were considered. However, the proportion of individuals with no risk-factor information has been increasing in recent years so current trends in diagnoses by risk factor may not be reliable. As most known cases of HCV in Ireland are in defined risk groups, the prevalence of HCV infection in the general population is thought to be very low. The Irish Blood Transfusion Service (IBTS) detected HCV in 0·02% of new blood donors between 1997 and 2008 (personal communication: Dr J. O'Riordan, IBTS, April 2010). Although blood donors are a very low risk group for HCV, we would expect a higher prevalence in this population if there was a large pool of undiagnosed infected people outside these main risk groups.
There may, however, be a significant number of undiagnosed cases in the recent migrant population in Ireland. Between 2001 and 2010, 125 882 new work permits were granted in Ireland (Department of Jobs, Enterprise and Innovation [25]). Seventy-six percent (n=95 977) of successful applicants were from countries with an estimated HCV prevalence of over 2% [Reference Shepard, Finelli and Alter26]. There is no systematic health screening for work permit applicants and it is likely that there are a significant number of undiagnosed cases of HCV in this population. Asylum was granted to about 3700 people in the same time period [27]. However, a significant proportion of asylum seekers are likely to have been tested for HCV, so the number of undiagnosed people in this population is probably small.
The information on distribution of HCV genotypes in the population is relevant to planning of treatment services, as genotype is one of the most important factors in determining the efficacy of antiviral treatment [2]. The sustained virological response (SVR) for patients infected with genotypes 1 and 4 is much lower than for those infected with genotypes 2 or 3. Country-based genotype information is also valuable in contributing to the mapping of European distribution of HCV genotypes.
The trends described here reflect dates of initial diagnosis rather than time of infection. As primary HCV infection is asymptomatic in most patients, laboratory diagnosis may have occurred many years after infection. This is particularly true in the early years of testing with the implementation of lookbacks to identify those infected through contaminated blood and blood products and the expansion of drug-treatment services. In a separate study of Irish people infected through blood and blood products, a median gap of 17 years was found between infection and diagnosis. Most of the patients in that study had been infected for over 25 years by the end of 2008 and, of those who were chronically infected, 14% had developed cirrhosis [28].
Relatively large time lapses between infection and diagnosis are likely in the early years of testing for drug users. However, we would expect this to have decreased in recent years. Demand for healthcare relating to serious liver complications in this cohort is expected to intensify significantly in the next decade as the prevalence of those chronically HCV infected for 20–30 years increases. In addition, three studies of drug users in Ireland have found a high prevalence of problem alcohol consumption (38% [Reference Comiskey and Cox16], 35% [Reference Ryder29] and 41% [30]) in this population and alcohol is known to be a significant co-factor in the progression of HCV liver disease to cirrhosis and HCC [31].
Study limitations
These estimates of burden of disease are not adjusted for hepatitis C treatment. In a large Irish cohort infected with HCV through blood and blood products, 41% of chronically infected patients had been treated and 48% of these had achieved a SVR [28]. However, anti-viral treatment uptake in the larger group of people infected through drug use is likely to be low. One study of HCV RNA-positive patients attending GPs for methadone treatment found that only 3% had received anti-viral treatment [Reference Cullen15]. However, when hepatitis C treatment is offered in conjunction with drug addiction treatment, success rates have been very good. The Drug Treatment Centre Board has treated about 14% of its 500 HCV RNA-positive patients to date and has achieved SVR rates of about 90% in genotype-3 patients and 55% in genotype-1 patients (personal communication: Dr S. Keating, The Drug Treatment Centre Board, March 2011).
Every effort has been made to remove duplicates and check the matches made. However, some patients may have been erroneously matched, or conversely some duplicate patients may still exist in the database. This is more likely to happen for patients whose records contain insufficient demographic information to allow matching. Data for infants were particularly sparse as specimens were often submitted without full names and other details.
CONCLUSIONS
There are a large number of people living with chronic HCV infection in Ireland and although the number of new diagnoses appears to be declining, significant numbers of cases continue to be notified each year. The information presented here will be of immense value in the future planning of health services for those with chronic HCV infection and will contribute to the overall understanding of the magnitude of the hepatitis C epidemic in Europe.
ACKNOWLEDGEMENTS
The authors are grateful to the following for their assistance with this project: Professor Joe Barry, Trinity College; Ms. Joanne Moran, NVRL; Dr Jean Long, Health Research Board; Dr Karina Butler, Our Lady's Children's Hospital, Crumlin; Dr Shay Keating, Drug Treatment Centre Board; Dr Joan O'Riordan, Irish Blood Transfusion Service.
DECLARATION OF INTEREST
None.







