Introduction
There is increasing scientific evidence of the existence of a relationship between diets rich in natural antioxidants and the prevention of several chronic diseases. Epidemiological studies have shown inverse associations between total dietary antioxidant intake and inflammation(Reference Brighenti, Valtueña and Pellegrini1, Reference Valtueña, Pellegrini and Franzini2), ischaemic stroke(Reference Del Rio, Agnoli and Pellegrini3), alterations in endothelial function(Reference Franzini, Ardigò and Valtueña4) and gastric cancer(Reference Serafini, Jakszyn and Luján-Barroso5). Moreover, adherence to a Mediterranean diet, which implies a higher plasma concentration of dietary antioxidants(Reference Bravo, Abia and Saura-Calixto6), is associated with a decrease in overall mortality and particularly in mortality resulting from CVD or cancer(Reference Sofi, Cesari and Abbate7).
Dietary antioxidants are a diverse group of chemical compounds, with varying solubility in biological fluids or biomembranes, and mechanisms of actions; they include carotenoids, vitamins C and E, and polyphenols(Reference Halliwell8). Of these, polyphenols (which include a wide variety of chemical structures that share one or more phenol groups) are the most commonly consumed dietary antioxidants, corresponding to about 90 % of dietary antioxidant intake(Reference O'Neill, Carroll and Corridan9, Reference Saura-Calixto and Goñi10).
The interest in polyphenol research within the fields of nutrition and food science has led to an increasing literature dealing with: (a) the bioavailability and distribution in tissues of polyphenols(Reference Manach, Williamson and Morand11); (b) possible mechanisms of action of polyphenols(Reference Ramos12); and (c) the health effects derived from polyphenol intake(Reference Scalbert, Manach and Morand13). In particular, clinical trials and/or epidemiological studies have shown that polyphenols play a role in the prevention of CVD and of certain kinds of cancer(Reference Hooper, Kroon and Rimm14, Reference Rossi, Bosetti and Negri15).
Current research into polyphenols focuses mainly on only a fraction of dietary polyphenols, corresponding to those that can be extracted from food with aqueous–organic solvents. The polyphenols identified in such extracts – named extractable polyphenols (EPP) – are then considered to be the total polyphenol content, and are used as the basis for calculations of dietary intake, bioavailability studies and to design intervention or observation studies.
However, it is known that a significant fraction of food polyphenols remains in the corresponding residues after the extraction; the so-called non-extractable polyphenols (NEPP)(Reference Bravo, Abia and Saura-Calixto6). Since foodstuffs are consumed as a whole, i.e. including both EPP and NEPP, significant amounts of NEPP are ingested daily, contributing to the reported health effects of polyphenols. However, this fraction of dietary polyphenols has long been neglected and studies of it are still scarce. The present review aims to provide an overview of dietary NEPP: their nature, occurrence in the diet, metabolic fate and possible health effects.
Non-extractable polyphenols
Several classifications for the different polyphenol classes, focused exclusively on EPP, have been proposed based on the different chemical skeletons that they present. One of the most common classifications divides them into flavonoids, phenolic acids, stilbenes, lignans and other polyphenols (including tyrosols and alkylresorcinols). All of them possess at least one aromatic ring, with one or more hydroxyl moieties. Several procedures have been reported for extracting polyphenols from plant foods; the most common is solid–liquid extraction with different combinations of organic solvents (methanol, ethanol or acetone) with water. Since there is abundant literature on EPP (more than 30 000 references according to the Thomson Reuters (formerly ISI) Web of Knowledge website; http://wokinfo.com/), including aspects such as their chemical characterisation, bioavailability, nutritional qualities and effects on health, this class of polyphenols is beyond the scope of the present review.
When performing such chemical extractions on foodstuffs, a solid residue remains. In polyphenol research, the residue is usually ignored and considered to be devoid of polyphenols. However, the residue is actually a rich source of another fraction of polyphenols with specific biological activities: NEPP.
NEPP are those dietary polyphenols that, after ingestion, are not significantly released from the food matrix either by mastication, an acidic pH in the stomach or the action of digestive enzymes. They reach the colon nearly intact, where they are subjected to extensive transformation by colonic microflora (see ‘Non-extractable polyphenols through the human gut’ section). NEPP include macromolecules, such as high-molecular-weight proanthocyanidins, and single phenolic compounds, such as phenolic acids, associated with macromolecules, mainly polysaccharide constituents of dietary fibre (DF) and protein. They are generally not included in polyphenol analysis of foodstuffs, although their NEPP content may be even higher than that of the EPP fraction (see ‘Occurrence of non-extractable polyphenols in foodstuffs and diets’ section). With regards to their chemical nature, NEPP comprise mainly polyphenols also found as EPP, such as proanthocyanidins, other flavonoids, phenolic acids and hydrolysable tannins(Reference Pérez-Jiménez and Torres16).
NEPP interact with the food matrix (mainly polysaccharides and proteins) via different mechanisms: (a) hydrogen bonding, as described for non-extractable proanthocyanidins (NEPA)(Reference Le Bourvellec, Bouchet and Renard17) and that may also be applicable to hydrolysable tannins; (b) hydrophobic interactions, including possible encapsulation into hydrophobic pockets with NEPA(Reference Le Bourvellec, Bouchet and Renard17); (c) covalent bonding (to form esters and ether bonds), in the case of phenolic acids(Reference Faulds and Williamson18–Reference Bunzel20), and possibly of NEPA and hydrolysable tannins. In addition, a fraction of EPP may be associated with NEPP(Reference Matthews, Mila and Scalbert21). Whatever the case regarding that last point, it should be pointed out that there are still many aspects related to the interactions, as well as to the physico-chemical structures of NEPP, that have yet to be elucidated.
Another important aspect of NEPP is that they are associated with DF and they can be considered to be constituents of DF. Since the first research at the end of the 1980s, which showed that this fraction of dietary polyphenols were constituents of DF(Reference Sauro-Calixto22, Reference Saura-Calixto, Goni and Manas23), in recent years some papers have emphasised the significant contribution of NEPP to the health-related properties attributed to DF polysaccharides(Reference Vitaglione, Napolitano and Fogliano24–Reference Saura-Calixto26). Therefore, NEPP do indeed contribute to some of the health-related properties commonly attributed to DF and EPP.
Occurrence of non-extractable polyphenols in foodstuffs and diets
Figure 1 shows the process followed for the analysis of the NEPP content of food(Reference Pérez-Jiménez, Arranz and Saura-Calixto27–Reference Arranz, Saura-Calixto and Shaha29). A chemical extraction with aqueous–organic solvents is carried out on the food, which releases EPP in the supernatant fraction and also produces a residue. The residue is then subjected to the action of digestive enzymes, to obtain a new residue containing NEPP. Chemical hydrolysis is then carried out, in order to release NEPP from the food matrix, or to fragment the polymers. The hydrolysates obtained after these treatments may then be analysed using spectrophotometric or chromatographic techniques. Although the NEPP content of food has been studied much less than the EPP content, the available data show the relevance of this fraction of dietary polyphenols.

Fig. 1 Scheme of the analysis of food non-extractable polyphenols (NEPP). EPP, extractable polyphenols; DAD, diode array detection.
Occurrence of non-extractable polyphenols in individual foodstuffs
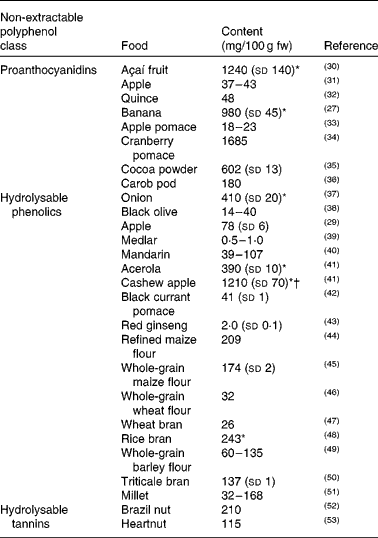
The different classes of NEPP are widespread among the different families of plant foods (cereals, fruit, vegetables, nuts, legumes), although some of them are particularly characteristic of a particular food family. Table 1(Reference Pérez-Jiménez, Arranz and Saura-Calixto27, Reference Arranz, Saura-Calixto and Shaha29–Reference Li, Tsao and Yang53) shows the reported content of NEPP belonging to different classes (proanthocyanidins, hydrolysable tannins and hydrolysable phenolics) in several plant foods.
Table 1 Non-extractable polyphenol content reported in the literature for several plant foods

fw, Fresh weight.
* Results expressed per 100 g dry weight.
† Spectrophotometric analysis, so may include gallic acid and/or ellagic acid derived from hydrolysable tannins.
NEPA are mostly found in fruit. Despite being obtained via different procedures, the data included in Table 1 provide a rough estimation of the NEPA content of foodstuffs. The case of the banana is particularly interesting, since its NEPA content (recently confirmed by an improved procedure(Reference Zurita, Díaz-Rubio and Saura-Calixto54)) would be about 100-fold the reported extractable proanthocyanidin content for this food(55). This reflects the contribution of this fraction of dietary polyphenols, that is usually ignored, to the total content of polyphenolic compounds in foodstuffs and therefore to dietary intake of such compounds.
Hydrolysable phenolics are comprised of several classes of phenolic compounds, although the most common are phenolic acids (ferulic acid, caffeic acid, sinapic acid and others). Although hydrolysable phenolics have usually only been studied in cereal products, they have recently been reported to be present in many other foodstuffs, such as onion, black olive, apple, medlar, mandarin, acerola, cashew apple, black currant pomace and red ginseng, as shown in Table 1.
Finally, hydrolysable tannins are more specific to certain foodstuffs. Ellagic acid and other derivatives from the hydrolysis of ellagitannins have been reported, for instance, to be present in several nuts (Table 1).
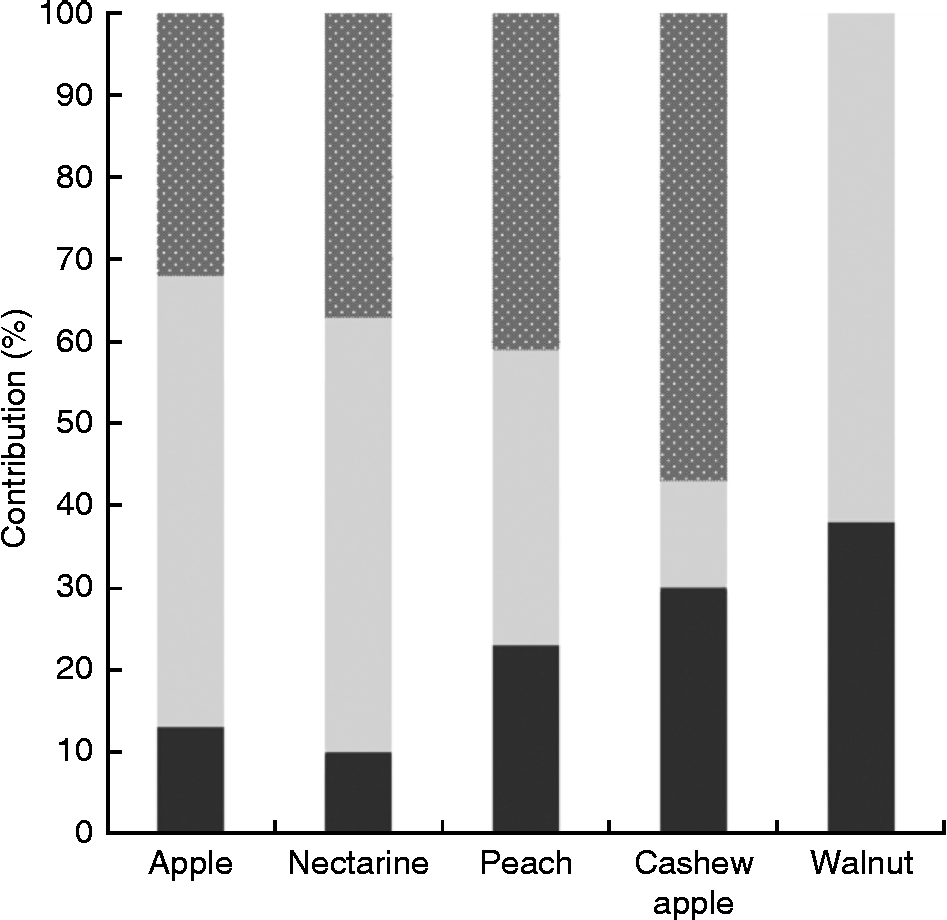
Another problem when it comes to evaluating the contribution of NEPP to the total polyphenol content of foodstuffs is that besides the fact that studies dealing with the NEPP content of foodstuffs are rare, they usually focus on only one class of NEPP (as is the case with most of the references included in Table 1) and do not consider the overall analysis of the different classes of NEPP. However, some methods have been proposed that include both the analysis of hydrolysable phenolics and NEPA in the evaluation of NEPP(Reference Arranz, Saura-Calixto and Shaha29, Reference Arranz, Silván and Saura-Calixto56); some of the results are shown in Fig. 2(Reference Arranz, Pérez-Jiménez and Saura-Calixto57). The analysis of EPP, hydrolysable phenolics and NEPA in several types of fruit and nuts showed that the contribution of NEPP was between 60 and 90 % of the total polyphenol content, and therefore represented the major fraction of these dietary antioxidants.

Fig. 2 Contribution of the different classes of non-extractable polyphenols to total polyphenol content in different foods(Reference Arranz, Saura-Calixto and Shaha29, Reference Rufino, Pérez-Jiménez and Tabernero41, Reference Arranz, Pérez-Jiménez and Saura-Calixto57). ![]() , Non-extractable proanthocyanidins;
, Non-extractable proanthocyanidins; ![]() , hydrolysable phenolics; ■, extractable polyphenols.
, hydrolysable phenolics; ■, extractable polyphenols.
Finally, since NEPP are constituents of DF, updated methods for the analysis of DF include analysis of NEPP(Reference Goñi, Díaz-Rubio and Pérez-Jiménez37). Such an approach has already been used in the analysis of DF from several types of fruit, vegetables and nuts(Reference Rufino, Pérez-Jiménez and Arranz30, Reference Goñi, Díaz-Rubio and Pérez-Jiménez37, Reference Rufino, Pérez-Jiménez and Tabernero41).
Occurrence of non-extractable polyphenols in diets
Analysis of NEPP in individual foodstuffs should serve as the basis for calculating NEPP content in whole diets in different populations; and therefore, for estimating NEPP intake from them. A few papers have dealt with the analysis and dietary intake of NEPP in the Spanish diet, as an example of the Mediterranean diet(Reference Saura-Calixto, Serrano and Goñi28, Reference Arranz, Silván and Saura-Calixto56) and in a rural Mexican population(Reference Hervert-Hernández, García and Rosado58).
As regards the Spanish diet(Reference Saura-Calixto, Serrano and Goñi28, Reference Arranz, Silván and Saura-Calixto56) when determining total polyphenol content in the different food consumed, it was found that in all the plant-based food groups (cereals, vegetables, legumes, fruit and nuts) the NEPP content was higher than that of EPP. When the data were translated into estimates of intake, total polyphenol intake in the Spanish diet was estimated to be about 1800–3000 mg/individual per d, depending on the analytical techniques used, with NEPP contributing about 50 % of the total polyphenol intake. Fruits were the highest contributor to NEPP intake (about 47 %), followed by cereals (31 %), vegetables (13 %), legumes (6 %) and nuts (4 %).
As the authors of the studies emphasised, some of the data are closer to estimates than to accurate determinations of NEPP intake, due to the analytical limitations. Nevertheless, the results constitute evidence of an appreciable presence of NEPP in plant foods and of their contribution to total polyphenol intake.
Another study focused specifically on the contribution of NEPP to polyphenol intake from fruit and vegetables in a rural Mexican population(Reference Hervert-Hernández, García and Rosado58). Despite the food items included in the categories in Mexico being very different from those consumed in Spain, it is remarkable that the results again showed that NEPP are more abundant in the solid plant-based food groups than EPP are and, therefore, they contribute significantly to the total polyphenol intake.
This kind of evaluation of total polyphenol content in diets, including NEPP, as well as estimations of the associated polyphenol intake, should be generalised to different populations as a starting point to advance our knowledge of the possible associations between NEPP intake and health effects that have not previously been considered.
Non-extractable polyphenols through the human gut
To understand the possible health effects associated with the intake of any bioactive compound, and therefore of NEPP, it is necessary to have knowledge of the different events that occur along the human gut, including:
(1) The solubilisation of the compounds in intestinal fluids, via different mechanisms, resulting in the compounds becoming bioaccessible. This may be estimated by determining the presence of NEPP in intestinal fluids, both in the small and in the large intestine.
(2) Possible transformations by colonic microbiota of the compounds that are bioaccessible in the colon. Such transformations may be enhanced or inhibited by the presence of other food components and/or other interactions with the microbiota. The metabolites derived from such transformations may be determined either in vitro (supernatant fractions from in vitro fermentation) or in vivo (caecal contents or faeces).
(3) Absorption, either of the original compounds or of the derived metabolites. Absorption gives rise to metabolites that may be determined in different biological fluids, such as urine and blood.
(4) Possible effects on target tissues. An accumulation of bioactive metabolites in certain tissues may contribute to the local effects of compounds of interest.
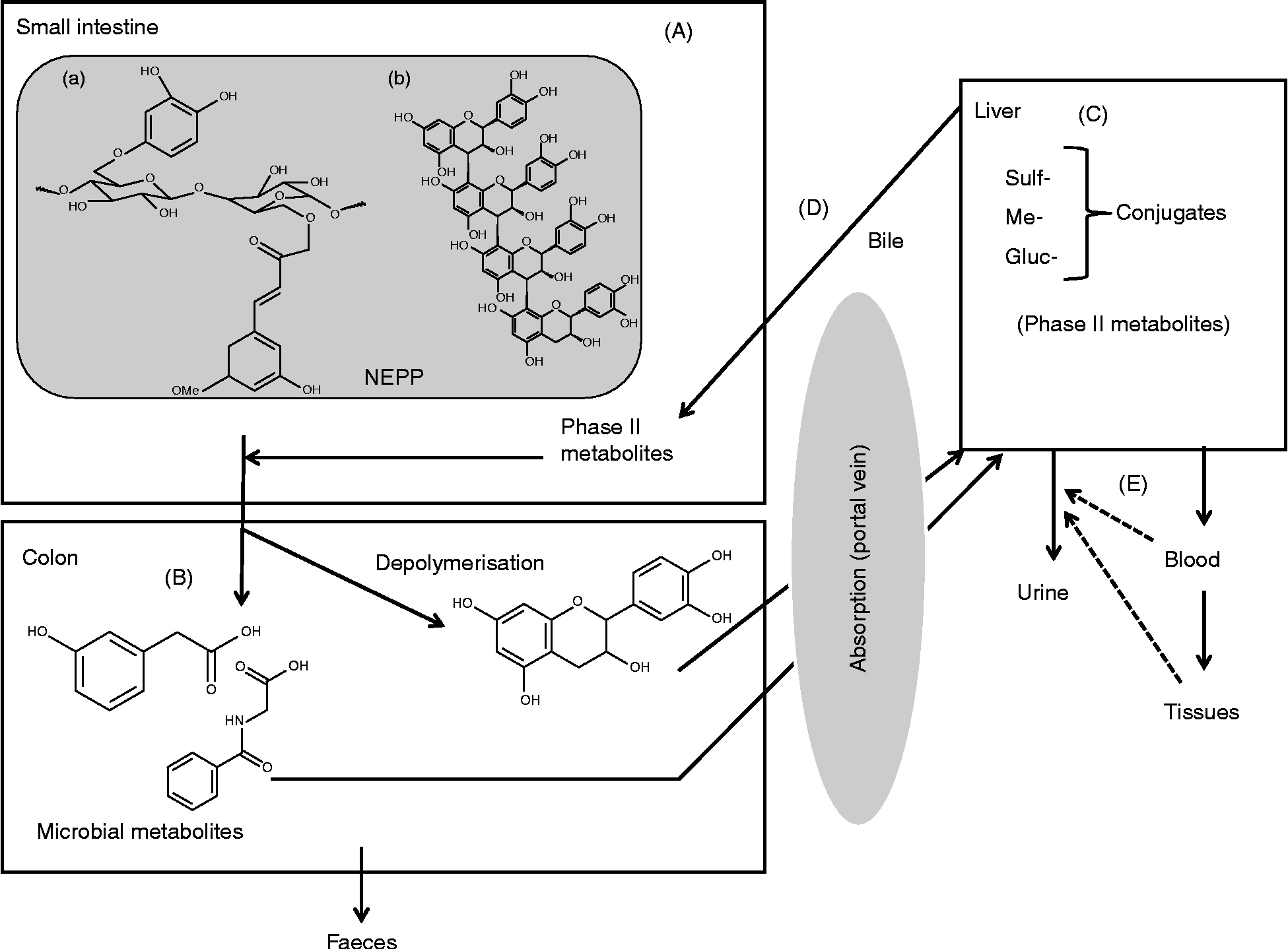
All these aspects have been studied in depth over recent years for EPP(Reference Manach, Williamson and Morand11), but studies on NEPP are scarce. Despite that, the available work that focuses on NEPP suggests that they are subjected to extensive transformation in the gut, giving rise to several absorbable metabolites that have been reported to have different health effects. From such work(Reference Saura-Calixto, Serrano and Goñi28, Reference Kroon, Faulds and Ryden59–Reference Mateos-Martín, Pérez-Jiménez and Fuguet65) a general scheme of the metabolic fate of NEPP can be proposed, as shown in Fig. 3, where compound ‘a’ represents a hydrolysable phenolic compound, i.e. a phenolic acid associated with a polysaccharide chain, and compound ‘b’ represents a polymeric NEPA. Briefly, most NEPP will pass through the small intestine (Fig. 3(A)) without any transformation. Once in the colon (Fig. 3(B)), and mainly through the action of the microbiota, NEPP are released and new compounds form. Some of these microbial metabolites may be absorbed though the portal vein, reaching the liver (Fig. 3(C)), where several related processes occur, giving rise to phase II metabolites. Once formed, these metabolites may return to the digestive tube through the bile (Fig. 3(D)), or pass into the bloodstream as a first step towards reaching the target tissues and finally be excreted in urine (Fig. 3(E)).

Fig. 3 Overview of the metabolic fate of non-extractable polyphenols (NEPP). Sulf-, sulfur; Me-, methyl; Gluc-, glucose.
Bioaccessibility of non-extractable polyphenols
Bioaccessibility corresponds to the amount of a food constituent that is solubilised in intestinal fluids as a consequence of physico-chemical conditions, or the action of either digestive enzymes or bacterial microbiota. Saura-Calixto et al. (Reference Saura-Calixto, Serrano and Goñi28) evaluated the bioaccessibility of total polyphenols, including NEPP, in a complete diet by using an in vitro gastrointestinal model followed by in vitro colonic fermentation. They observed that, while about a 50 % of hydrolysable phenolics become bioaccessible in the small intestine, in the case of NEPA most of them arrive nearly intact to the colon. Similarly, other studies have shown that NEPA are only partially depolymerised in the small intestine(Reference Touriño, Pérez-Jiménez and Mateos-Martín64, Reference Mateos-Martín, Pérez-Jiménez and Fuguet65). Once in the colon, those NEPP that are not solubilised in the small intestine become accessible either by: (a) fermentation by colonic microbiota of the molecules to which they are associated (carbohydrates or proteins); or (b) the action of some intestinal enzymes able to break covalent bonds, such as esterases(Reference Kroon, Faulds and Ryden59). It has been estimated that 95 % of NEPA arriving to the colon are released from the food matrix by these two mechanisms(Reference Saura-Calixto, Serrano and Goñi28).
Therefore, significant amounts of the different classes of NEPP appear in the large intestine daily as bioaccessible compounds, including both the compounds that have been solubilised in the small intestine but have not been absorbed, as well as those released in the large intestine. Since NEPP are widespread among the different groups of plant foods, a diverse diet including food from all of them would guarantee a continuous supply of beneficial bioaccessible compounds through the digestive tract. Obviously, a fraction of EPP not previously absorbed in the small intestine also arrives as bioaccessible compounds to the large intestine(Reference Saura-Calixto, Serrano and Goñi28); future work should elucidate the particular contributions of EPP and NEPP to the pool of bioaccessible phenolic compounds present in the large intestine.
Colonic transformation and bioavailability of non-extractable polyphenols
In the colon, NEPP released from the food matrix and polymeric NEPP undergo colonic fermentation, which produces potentially absorbable metabolites. Work focusing strictly on the fermentation of dietary NEPP is still scarce and mostly qualitative(Reference Touriño, Fuguet and Vinardell62–Reference Mateos-Martín, Pérez-Jiménez and Fuguet65), but it has already provided clear evidence on their colonic fermentation. For instance, supplementing rats with a NEPP concentrate devoid of EPP resulted in the presence of polyphenol-derived metabolites in the urine and faeces (ten and three, respectively) at concentrations at least 10-fold higher than in a non-supplemented group(Reference Mateos-Martín, Pérez-Jiménez and Fuguet65).
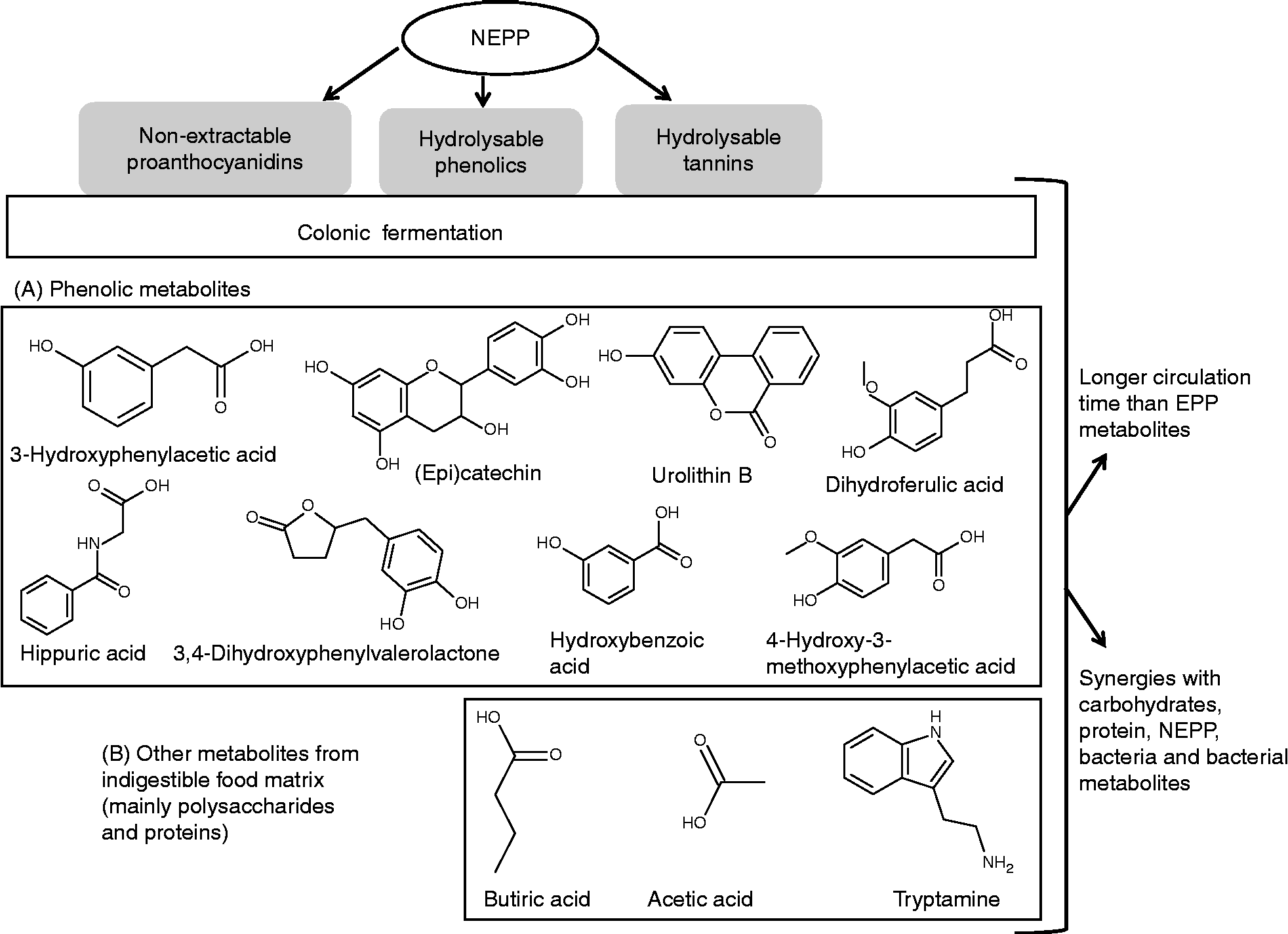
On the other hand, since many studies of the metabolic fate of polyphenols are based on supplementation with whole foods, many of which evidently contain NEPP, some of the data regarding the metabolites derived from polyphenols would actually correspond to NEPP metabolites. Therefore, considering specific studies on NEPP fermentation(Reference Touriño, Fuguet and Vinardell62–Reference Braune, Bunzel and Yonekura66) and other studies on foods containing both EPP and NEPP(Reference Cerdá, Espín and Parra67–Reference Selma, Espín and Tomás-Barberán69), these would be the main microbial metabolites derived from the different fractions of NEPP(Reference Touriño, Fuguet and Vinardell62–Reference Selma, Espín and Tomás-Barberán69):
(1) Metabolites derived from NEPA. These include: (a) the monomeric constituents of NEPA polymers, mainly (epi)catechin; and (b) a wide variety of compounds derived from the breakdown of the basic skeleton of flavanols and further degradation, which gives place first to valerolactones, then to several phenolic acids corresponding to successive degradations (hydroxyphenylpropionic acids, hydroxyphenylacetic acids, etc.) and then to small acids such as hippuric acid.
(2) Metabolites derived from hydrolysable phenolics. Although free phenolic acids released from the food matrix may be absorbed directly, they may also undergo additional transformation resulting from interaction with the colonic microbiota. For instance, ferulic acid is transformed into dihydroferulic acid, which is later metabolised to other phenolic acids coincident with the degradation of proanthocyanidins.
(3) Metabolites derived from hydrolysable tannins. Ellagic acid released from ellagitannins in the gastrointestinal tract is transformed by the microbiota into a particular class of compounds, the urolithins.
The presence of these compounds has been determined in caecal contents and in faeces(Reference Touriño, Pérez-Jiménez and Mateos-Martín64, Reference Mateos-Martín, Pérez-Jiménez and Fuguet65), but their determination is most common in urine and blood (in both animal studies and clinical trials)(Reference Touriño, Fuguet and Vinardell62, Reference Saura-Calixto, Pérez-Jiménez and Touriño63, Reference Braune, Bunzel and Yonekura66). The results show that these compounds are absorbed and therefore become bioavailable and can have different biological effects on the human body, as well as possible local effects on the colon (see ‘Health-related properties of non-extractable polyphenols’ section).
The metabolites described above do not differ from those described as derived from EPP colonic fermentation(Reference Manach, Williamson and Morand11, Reference Crozier, Jaganath and Clifford68), i.e. the colonic fermentation of both EPP and NEPP gives place to the same compounds. However, the specific characteristics of NEPP (high molecular weight and/or association with other macromolecules present in the food matrix) cause two specific features in the colonic transformation of NEPP as compared with EPP:
(1) The absorption of NEPP is retarded in relation to that of EPP, as shown by the delayed peak in plasma ferulic acid levels after the intake of wheat bran (containing NEPP) in comparison with that of pure ferulic acid(Reference Rondini, Peyrat-Maillard and Marsset-Baglieri60), or the delayed peak in plasma antioxidant capacity after the intake of a NEPA-rich matrix in comparison with the data for EPA-rich matrixes(Reference Ye, Chen and Liu40). Such data indicate that metabolites derived from NEPP circulate for longer periods in the human body than those derived from EPP. Indeed, some of the metabolites detected in the urine and faeces of rats fed with NEPA-rich products and in the supernatant fractions from in vitro fermentation of these matrixes were also detected in plasma from fasting volunteers after 24 h of a polyphenol-free diet, which demonstrates the prolonged circulation times of NEPA metabolites(Reference Saura-Calixto, Pérez-Jiménez and Touriño63). Therefore, NEPP may continually provide the colonic microbiota with a significant amount of fermentable substrates that need more time to be released and fermented than EPP due to their specific nature, and, therefore, result in a more prolonged circulation of beneficial metabolites.
(2) Since NEPP do not reach the colon alone, but in combination with other fermentable dietary components, such as proteins and polysaccharides, their fermentation rate may be affected. Indeed, some studies in rats have shown that the presence of DF enhances the fermentation of polyphenols(Reference Aprikian, Duclos and Guyot70, Reference Juśkiewicz, Milala and Jurgoński71). Moreover, in the work by Saura-Calixto et al. (Reference Saura-Calixto, Pérez-Jiménez and Touriño63), the in vitro fermentability (as SCFA production compared with a control) of a concentrate of polymeric NEPP was 23 %, while that of a concentrate of NEPP associated with DF was 50 %, probably due to an increase in bacterial activity. Therefore, the colonic fermentation of NEPP would be accompanied by a synergism with the other components of the food matrix to which they are associated, an aspect that is not present in the fermentation of EPP, which are free in the food matrix.
A summary of some of the colonic metabolites derived from NEPP, as well as the specific features of their fermentation, is shown in Fig. 4. Nevertheless, more specific work on the colonic metabolism of NEPP from different origin and/or different nature, including quantitative data, is needed. This would allow elucidation of the relative contributions of EPP and NEPP to the dozens of polyphenol-derived metabolites that circulate in the human body after plant food intake, and that may be indeed be responsible for many of the reported benefits of polyphenols(Reference Selma, Espín and Tomás-Barberán69).

Fig. 4 Main features of non-extractable polyphenol (NEPP) colonic fermentation. EPP, extractable polyphenols.
Health-related properties of non-extractable polyphenols
As previously described, NEPP undergo extensive transformation in the colon. Therefore, the health effects associated with them may not come mainly from the intact NEPP, but from their metabolites. In recent years, a number of studies have started to focus on the biological activities of polyphenol metabolites. Although they do not specifically consider the contribution of NEPP to their release, a significant proportion of the production of such compounds in the human body, and therefore of their associated effects, would be due to NEPP intake, as explained above.
Table 2(Reference Schroeter, Heiss and Balzer72–Reference González-Sarrías, Espín and Tomás-Barberán76) summarises some of the findings regarding the biological effects of these metabolites; the results are mostly obtained in cell cultures and animal models. The effects include anti-inflammatory activity, a reduction of oxidative stress, the inhibition of protein glycation, antiproliferative effects and effects on flow-mediated vasodilation(Reference Schroeter, Heiss and Balzer72–Reference González-Sarrías, Espín and Tomás-Barberán76). These are probably the mechanisms that underlie the observed health effect of NEPP-rich products in animal and human studies.
Table 2 Summary of biological effects described for polyphenol metabolites

COX-2, cyclo-oxygenase-2; DSS, dextran sodium sulfate.
Moreover, since a significant fraction of NEPP is present in foods associated with polysaccharides, when such a complex reaches the colon, the polysaccharide fraction also ferments, releasing beneficial components such as butyric acid, associated with the prevention of colorectal cancer(Reference Velázquez, Lederer and Rombeau77, Reference Wong, De Souza and Kendall78), and acetate and propionate, with potential health effects on lipid metabolism(Reference Wong, De Souza and Kendall78, Reference Hosseini, Grootaert and Verstraete79). These metabolites also contribute to the health effects reported in animal or human studies of NEPP-rich products.
Gastrointestinal health
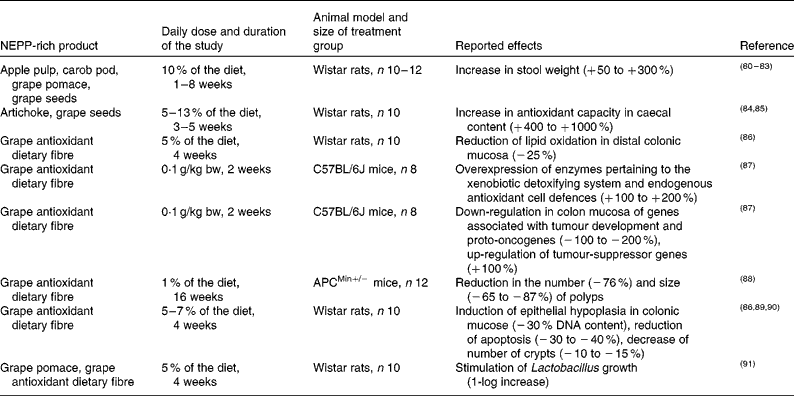
Since NEPP reach the colon nearly intact and that is where they suffer most of their metabolic transformation, it is evident that one of their major targets of action is gastrointestinal health. Several studies have shown promising results in this field after supplementation of different animal models with several NEPP-rich products (Table 3)(Reference Bravo, Saura-Calixto and Goni80–Reference Pozuelo, Agis-Torres and Hervert-Hernández91). Grape antioxidant DF, a matrix derived from wine production with a high content of NEPA(Reference Hervert-Hernández, Pintado and Rotger92), mostly associated with DF, has been the most commonly tested matrix; however, some studies have also fed rats with apple pulp, carob pod, artichoke, grape pomace or grape seeds. Most studies included ten animals per group (commonly Wistar rats) and carried out the supplementation by including a 5–13 % of the tested product in the diet. The duration of the study was commonly 4 weeks.
Table 3 Summary of animal studies on the effect of non-extractable polyphenols (NEPP) on gastrointestinal health

bw, Body weight.
Overall, these animal studies show that NEPP may have a beneficial effect on gastrointestinal health through a combination of mechanisms(Reference Bravo, Saura-Calixto and Goni80–Reference Pozuelo, Agis-Torres and Hervert-Hernández91):
(1) An increase in stool weight, reducing transit time and therefore the time of contact of toxic compounds with the colonic epithelium(Reference Bravo, Saura-Calixto and Goni80–Reference Bravo, Mañas and Saura-Calixto83).
(2) An increase in the antioxidant capacity of caecal content and in the expression of endogenous antioxidant systems, thus counteracting the tumour-promoting reactive oxygen species (ROS) present in the colon(Reference Goñi, Jiménez-Escrig and Gudiel84, Reference Goñi and Serrano85, Reference López-Oliva, Agis-Torres and García-Palencia89, Reference López-Oliva, Agis-Torres and Goñi90).
(3) A prebiotic effect proven by stimulation of Lactobacillus, which would also imply a reduction in the proportion of non-beneficial bacteria species(Reference Pozuelo, Agis-Torres and Hervert-Hernández91). This prebiotic effect was observed when rats were supplemented with a NEPP-rich product but not when their diet contained the same amount of cellulose, indicating that this was a specific effect of NEPP and not of DF. Previous in vitro studies had also reported a prebiotic effect for grape pomace, another NEPP-rich product(Reference Hervert-Hernández, Pintado and Rotger92).
(4) An increase in antiproliferative capacity in healthy rats, shown by an induction of epithelial hypoplasia, reduction of apoptosis and a decrease in the number of crypts(Reference López-Oliva, Pozuelo and Rotger86, Reference López-Oliva, Agis-Torres and García-Palencia89, Reference López-Oliva, Agis-Torres and Goñi90). These antiproliferative effects were additionally found in cell cultures treated with a NEPP concentrate from apple pomace(Reference Aprikian, Duclos and Guyot70).
(5) A reduction of intestinal tumorigenesis in APCMin/+ mice, an animal model of colorectal cancer, including significant reductions in the number ( − 76 %) and size ( − 65 to − 87 %) of polyps(Reference Sánchez-Tena, Lizárraga and Miranda88).
(6) Modifications in gene expression in healthy mice and in APCMin/+ mice, in particular, down-regulation of genes associated with tumour development and proto-oncogenes (for example, genes belonging to the RAS family, such as RASSF4, RAP2C and RAP2B), and up-regulation of tumour-suppressor genes (for instance, NLB1 or TGFb3, related to cell cycle and cell proliferation, respectively)(Reference Lizarraga, Vinardell and Noé87, Reference Sánchez-Tena, Lizárraga and Miranda88).
In none of these studies neither adverse nor toxic effects were observed in the animals.
Overall, all these animal studies have shown a promising role for NEPP in the prevention and/or treatment of colorectal cancer and other gastrointestinal disorders, which should be validated in human subjects with proper clinical trials. Interestingly, when grape antioxidant DF was provided to human subjects, an increase in weekly faecal outputs was observed in those subjects who initially exhibited seven or fewer faecal outputs per week(Reference Pérez-Jiménez, Serrano and Tabernero93). Although grape antioxidant DF is a DF-rich product and this bulking effect is well known for DF, it should be emphasised that NEPP are indeed constituents of DF(Reference Saura-Calixto26), although they are usually not considered, and they may make a specific contribution to this bulking effect.
Also, a recent epidemiological study(Reference Rossi, Bosetti and Negri15) showed an inverse association between proanthocyanidin intake and the risk of colorectal cancer; furthermore the reduction in risk was greater, the higher the degree of polymerisation of the proathocyanidins (> 10). This study only considered the intake of EPA, but since NEPA are also present in common foodstuffs and they consist of large polymeric structures, these results highlight the need to corroborate the possible role of NEPP – and in particular NEPA – in the prevention of colorectal cancer.
CVD risk factors
The absorption of bioactive metabolites generated in the colon (both from the fermentation of NEPP themselves and from that of their associated polysaccharides) may give rise to systemic effects that manifest in other organs and tissues. In particular, the possible role that these colonic metabolites may play in the prevention of CVD has been expressed as the ‘gut–heart axis’ hypothesis(Reference Vitaglione, Fogliano, Van Der Kamp, Jones, McCleary and Topping94). Several studies in animal models and/or human subjects(Reference Martín-Carrón, Saura-Calixto and Goñi82, Reference Lizarraga, Vinardell and Noé87, Reference Pérez-Jiménez, Serrano and Tabernero93, Reference Robertson95) have shown that a NEPP-rich matrix may mitigate certain risk factors for CVD, such as hyperlipidaemia or hypertension. As regards to the effects in lipidaemia, this could occur through different mechanisms, such as a reduction in lipid biosynthesis (proven by a down-regulation of the expression of genes involved in this process) and an increase in faecal excretion of cholesterol(Reference Martín-Carrón, Saura-Calixto and Goñi82, Reference Bravo, Mañas and Saura-Calixto83, Reference Lizarraga, Vinardell and Noé87). Moreover, NEPP could also be related to the prevention of CVD by a reduction of lipid oxidation(Reference Pérez-Jiménez, Serrano and Tabernero93), which has shown to be associated with CVD(Reference Lecumberri, Goya and Mateos96).
Also, it should be considered that the association of NEPP with DF may give rise to specific synergies. In this way, the daily supplementation to hypercholesterolaemic subjects with 7·5 g of a grape-derived NEPP-rich product for 16 weeks resulted in significant reduction in plasma cholesterol and blood pressure, which were greater than those described separately for DF and EPP in several meta-analyses. This may be due to the combined effects of a single matrix containing DF and associated NEPP(Reference Hervert-Hernández, Pintado and Rotger92).
Finally, a field which has so far not been explored and in which NEPP may have a preventive effect is that of the metabolic syndrome and, in particular, in the maintenance of homeostatic glucose levels through action on insulin sensitivity. The existence of metabolic crosstalk between the colon and periphery organs affecting insulin sensitivity has been suggested(Reference Cracowski and Ormezzano97). More recently, the supplementation of mice with grape antioxidant DF produced a decrease in blood glucose, compared with controls, and up-regulation of the gene encoding the enzyme glucose-6-phosphatase, which plays a key role in the homeostatic regulation of blood glucose(Reference Lizarraga, Vinardell and Noé87).
Concluding remarks
NEPP constitute an important fraction of dietary polyphenols, which has commonly been ignored in polyphenol research.
The different classes of NEPP are widespread among all the families of plant foods, and their content in many foodstuffs is higher than that of EPP. Therefore, they significantly contribute to polyphenol intake, which is commonly considered to be derived only from EPP.
Studies of the metabolic fate of NEPP, although scarce, have shown that NEPP are extensively fermented in the colon, releasing several bioactive and absorbable metabolites.
The main site of action of NEPP is the colon, with several studies of different animal models showing different mechanisms by which NEPP may play a preventive role with regard to colorectal cancer. Also, NEPP (through their bioactive metabolites) may also play a role in the prevention of other chronic diseases, in particular in mitigating certain risk factors for CVD.
The health properties of DF and phenolic compounds are commonly attributed to polysaccharides and EPP, respectively; in both cases this disregards the relevant contribution of NEPP to such properties.
Further research into NEPP should be encouraged, including analysis of food content, estimates of dietary intakes in different populations, studies of their metabolic fate and sites of action, and evaluation of their health effects through clinical trials. This would help us to gain a complete overview of the biological and nutritional relevance of this understudied fraction of dietary antioxidants.
Acknowledgements
J. P.-J. acknowledges the Spanish Ministry of Science and Innovation for granting her a ‘Juan de la Cierva’ postdoctoral contract. M. E. D.-R. acknowledges the CSIC (Spanish Research Council) for granting her a ‘JAE-Doc’ postdoctoral contract. The present review was supported by the Spanish Ministry of Science and Innovation (project AGL2011–27741). The Spanish Ministry of Science and Innovation and the CSIC had no role in the design, analysis or writing of this article.
F. S.-C. planned the review and designed the manuscript, jointly with J. P.-J. The first version of the manuscript was written by J. P.-J., supervised by F. S.-C. All the authors contributed to writing the manuscript and approved the final version.
None of the authors had any conflict of interest.