During the last decade vitamin D has emerged from the shadow of other nutrients to centre stage. This trend has been driven by a combination of factors including recent disappointing negative findings from supplementation trials of other vitamins(Reference Byers1), some of which have shown harm(Reference Bjelakovic, Nikolova and Gluud2), and the evidence of pleiotropic effects of vitamin D on many body tissues(Reference Norman and Bouillon3). A further development has been the report from the US Institute of Medicine (IOM) revising slightly higher the recommended daily intake of vitamin D(4), which has received a mixed response from researchers and clinicians(Reference Heaney and Holick5, Reference Reid and Avenell6). The accumulating evidence, much of which was ignored by the IOM because of its observational nature, has reached tipping point for a number of disease outcomes, such that randomised controlled trials (RCT) are required urgently to provide certainty about the possible health benefits from vitamin D.
The aims of the present overview are to summarise the research on common diseases for which there is substantial evidence on vitamin D and identify diseases where vitamin D may be beneficial. The focus is on community-based studies of free-living people, rather than studies of clinic-based patients or laboratory-based animals, which are cited only to understand possible biological mechanisms; and on vitamin D itself, rather than the active metabolite calcitriol and other analogues of active vitamin D. Such evidence is required to support the introduction of public health programmes to increase the vitamin D status of the general population, which, surely, should be our ultimate aim, should vitamin D be shown by RCT to be beneficial.
Study design and causation
The quality of the evidence used to decide whether vitamin D is beneficial depends on study design. At the lowest rung sit ecological studies, which examine associations between exposure (e.g. vitamin D status) and disease at the group level. Typically, comparisons of these measures are made for populations living in towns, cities, counties or countries. These studies are typically used to generate rather than test hypotheses, since the association between exposure and disease is not made at the individual level. The latter studies, where exposure and disease status are measured in individuals, are termed analytic and are categorised into two main groups: (i) experimental studies, such as RCT, where the exposure is actively changed by the researchers; and (ii) observational studies, where the researchers only observe and measure exposure.
Epidemiologists typically use the following hierarchy, in descending order of importance, to decide on causation when comparing results from different analytical study designs: (i) experimental studies (i.e. RCT) provide the strongest evidence, since the exposure is actively modified by researchers; (ii) cohort studies (including nested case–control studies) are next, since measurement of exposure precedes disease onset; and (iii) case–control and cross-sectional studies are lowest, since measurement of exposure may be affected by the disease process or biased after disease onset.
Vitamin D status
Vitamin D occurs in the human body in two forms, either as: (i) cholecalciferol (vitamin D3) from sun exposure or eating animal foods; or (ii) ergocalciferol (vitamin D2) from mushrooms and yeast irradiated with UV light(Reference Holick7). The sun is the major source of vitamin D in most land animals including man, and vitamin D3 is synthesised in the skin by UV-B radiation activating its precursor 7-dehydrocholesterol(Reference Holick7); although dietary sources of vitamin D can also be important, particularly in people who take vitamin D supplements(Reference Garland, French and Baggerly8). Vitamin D from both sources then circulates in the blood to the liver where it is converted to its main metabolite, 25-hydroxyvitamin D (25(OH)D), which has blood levels about 1000 times higher than the active metabolite, 1,25-dihydroxyvitamin D (1,25-(OH)2D). Until recently, it was thought that the conversion to 1,25-(OH)2D occurred only in the kidneys, but there is now overwhelming evidence, originally from laboratory cancer research(Reference Schwartz, Whitlatch and Chen9), that the cells of most organs have the vitamin D receptor and, along with this, the capacity to synthesise 1,25-(OH)2D locally – the so-called autocrine or paracrine synthesis of the active metabolite(Reference Norman and Bouillon3, Reference Peterlik and Cross10). The autocrine synthesis of 1,25-(OH)2D is dependent on circulating levels of 25(OH)D. The main marker of vitamin D status is 25(OH)D, which provides a better assessment of vitamin D status than dietary methods since most vitamin D comes from sun exposure(Reference Haddad and Hahn11, Reference Poskitt, Cole and Lawson12). Objective laboratory measurements of blood vitamin D levels are likely to be less biased than measurement of dietary vitamin D intake using subjective questionnaire methods. Thus, cohort studies which associate 25(OH)D levels at baseline with subsequent disease risk provide the strongest evidence of causation next to that from RCT of vitamin D supplementation.
Vitamin D and disease
Vitamin D has been linked to a very wide range of diseases, but sufficient evidence from epidemiological studies has now emerged for the following diseases: fractures, cancer, CVD (including hypertension and type 2 diabetes), and diseases from altered immune function. The evidence for each is discussed in turn, with the focus on cohort studies of non-clinic selected samples with baseline blood measures of 25(OH)D and RCT of vitamin D supplementation.
Methods
To identify relevant articles for each of these disease groups, PubMed was searched for the years 2000 to 2010 (19 March 2011) using the term ‘vitamin D’ with the following keywords: ‘fractures’ or ‘osteoporosis’ (number of publications=2161); ‘cancer’ (=2031); ‘infection’ or ‘cytokines’ for immune function (=955); and ‘cardiovascular disease’ (=736). The annual number of publications in each of these four disease categories during 2000–2010 is shown in Fig. 1. Cohort studies and RCT were identified by searching the abstracts of these publications, and recent reviews and meta-analyses were searched to identify studies published before 2000. The inclusion criteria for cohort studies (including nested case–control studies) were: (i) study samples recruited from community or occupational groups (patient samples were excluded); and (ii) baseline vitamin D status assessed using blood measures of 25(OH)D (studies of dietary vitamin D status were excluded). Many of the cohort studies of CVD also reported on all-cause mortality, so this has been added as an additional outcome in the overview. Eligible cohort studies are summarised in Tables 1–3 for fractures (n 9), colorectal cancer (n 10), CVD (n 13) and all-cause mortality (n 12).
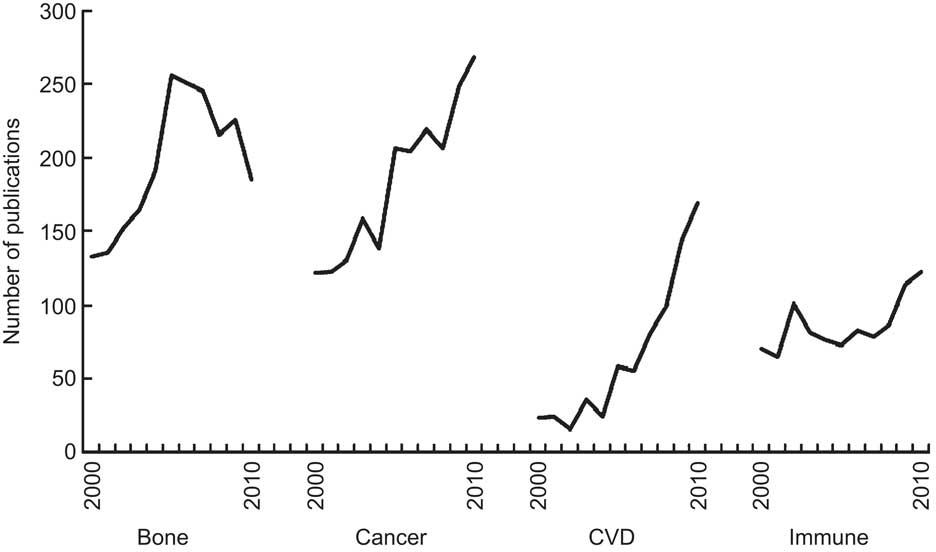
Fig. 1 Trends in vitamin D publications, by disease group, 2000 to 2010
Table 1 Relative risk of having a fracture associated with low baseline level of 25-hydroxyvitamin D (25(OH)D) in cohort studies
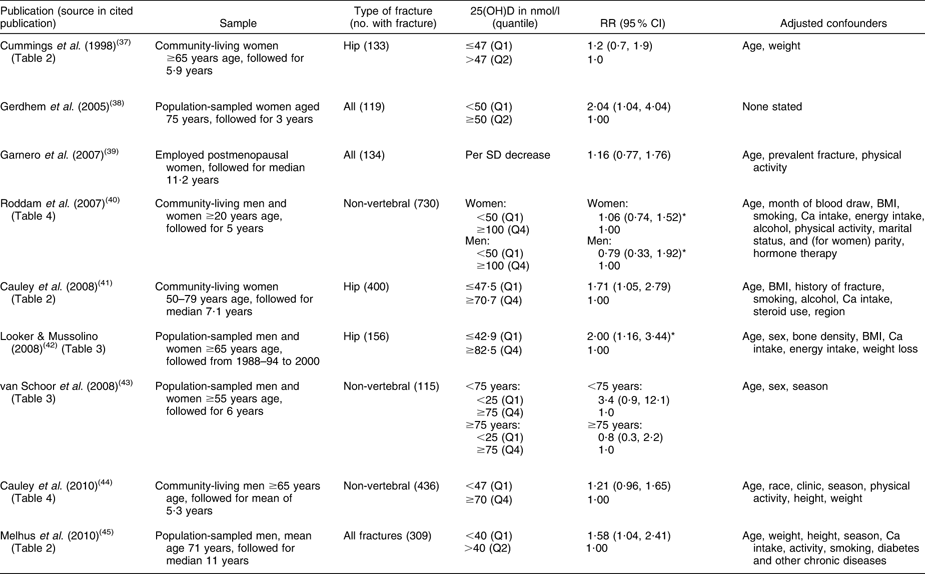
RR, relative risk.
*Inverse of published relative risk to make highest 25(OH)D group the reference.
Table 2 Relative risk of having colorectal cancer associated with low baseline level of 25-hydroxyvitamin D (25(OH)D) in cohort studies
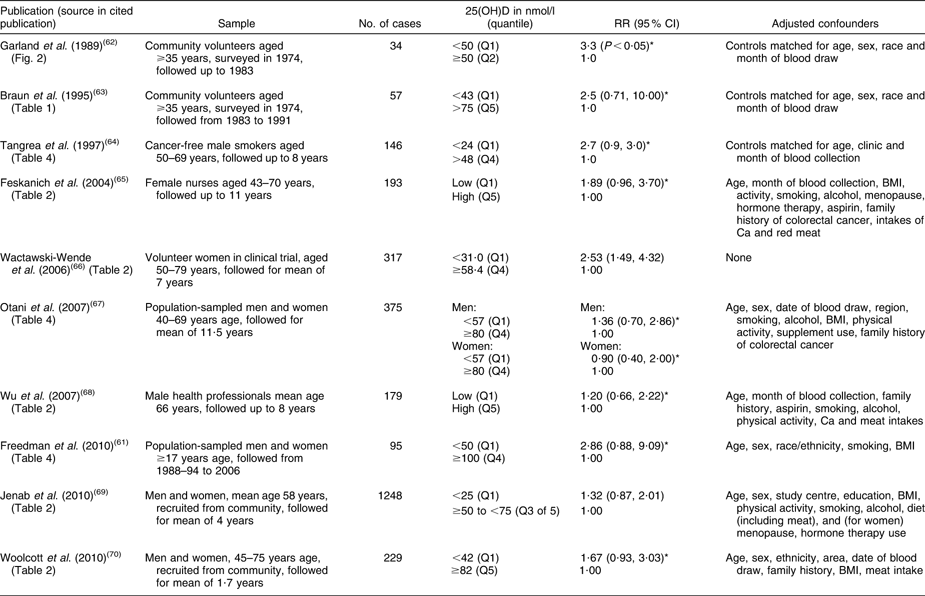
RR, relative risk.
*Inverse of published relative risk to make highest 25(OH)D group the reference.
Table 3 Relative risk of CVD and all-cause mortality associated with low baseline level of 25-hydroxyvitamin D (25(OH)D) in cohort studies

RR, relative risk; MI, myocardial infarction; SES, socio-economic status.
*Inverse of published relative risk to make highest 25(OH)D group the reference.
†Fatal and non-fatal disease.
‡Fatal disease only.
§Confidence intervals estimated from Fig. 3.
∥Non-fatal disease only.
Measures of effect (relative risks, odds ratios and hazard ratios) associated with quantiles of 25(OH)D were extracted from identified cohort studies and summarised with RevMan software version 5·1 (The Nordic Cochrane Centre, Copenhagen), using a random effects model with weighting by the inverse variance method and the I 2 test to assess heterogeneity(13). This approach of summarising effect measures from 25(OH)D quantiles has been used in previous meta-analyses(Reference Pittas, Chung and Trikalinos14, Reference Grandi, Breitling and Brenner15). The main advantages of this approach are that: (i) non-linear associations can be evaluated (this issue is discussed later with regard to the IOM report and the public health implications of current research); and (ii) any effect from variation between different methods for measuring 25(OH)D is minimised as the relative risks come from comparisons of participants tested with the same assay within each study. However, the main disadvantage is that the cut-off points used to define 25(OH)D categories vary between studies, which may have resulted in attenuation of effect because of measurement error. Further, the cut-off points in many studies have been below the range of 80–100 nmol/l associated with optimum health outcomes(Reference Bischoff-Ferrari, Giovannucci and Willett16), which also will have resulted in underestimation of the maximum effect measure. Thus, the summary measures of effect in the present report probably underestimate the full effects associated with variations in 25(OH)D across the normal population range.
Fractures
Recognition of the role of vitamin D deficiency in causing bone disease extends back to the 1920s, from the classic studies showing that vitamin D supplementation cured rickets(Reference Chick17). Evidence that vitamin D deficiency might have a causal role in fractures first came from UK studies carried out in the 1970s which showed that osteomalacia (‘adult rickets’) was common in patients with hip fracture(Reference Aaron, Gallagher and Anderson18) and more common after winter (February–April) than in other months of the year(Reference Aaron, Gallagher and Nordin19). Following this, numerous (at least thirty) case–control studies were carried out which collectively showed that hip-fracture cases had lower 25(OH)D levels than controls(Reference Weatherall20).
The usual sequence is for cohort studies to be carried out after case–control studies, before progressing to RCT. However, for fractures, the RCT were started first, partly driven by pharmaceutical companies with a commercial interest, seeking to show that the active metabolite (calcitriol) and its analogues (alphacalcidol) might reduce the risk of fractures. At least twenty-five studies of active vitamin D have been carried out, extending back to the 1980s; with meta-analyses of these showing that active vitamin D reduces the incidence of fractures, but increases the incidence of hypercalcaemia(Reference Richy, Ethgen and Bruyere21–Reference Avenell, Gillespie and Gillespie23). This latter finding, combined with the high cost of active vitamin D, which needs to be taken daily because of its short half-life, make these medications unsuitable for population-based fracture prevention programmes.
At least twenty-four RCT have been carried out using vitamin D (alone or with Ca)(Reference Avenell, Gillespie and Gillespie23), beginning with the first one from France published in 1992(Reference Chapuy, Arlot and Duboeuf24) and continuing to the most recent in 2010 from Australia and Finland(Reference Sanders, Stuart and Williamson25, Reference Salovaara, Tuppurainen and Karkkainen26). These RCT have two widely accepted limitations. First, the daily dose in many of these studies (average about 12·5 μg/d) is now considered too low as it would raise 25(OH)D levels only by 10–15 nmol/l(Reference Heaney27). Current recommendations are that at least 42·5 μg/d is required to increase 25(OH)D levels up to those associated with optimum health (80 nmol/l)(Reference Vieth, Bischoff-Ferrari and Boucher28). Second, many of these RCT gave Ca in combination with vitamin D, and usually with higher doses of vitamin D (17·5–20 μg/d) compared with studies that gave vitamin D by itself (10 μg/d). Thus, it is not possible to conclude whether any beneficial effect on fracture incidence is from vitamin D by itself. Hence the inconsistent findings from recent meta-analyses, with some concluding that vitamin D is beneficial against fractures only when combined with Ca(Reference Avenell, Gillespie and Gillespie23, Reference Boonen, Lips and Bouillon29, 30) and others concluding that vitamin D taken in higher doses (>17·5 μg/d) is effective by itself(Reference Bischoff-Ferrari, Willett and Wong31–Reference Sawka, Ismaila and Cranney34), while yet another concluded that Ca supplementation by itself had no effect on fracture incidence(Reference Bischoff-Ferrari, Dawson-Hughes and Baron35). The eventual outcome of this debate has important implications for any future public health prevention strategies, should future research show that vitamin D (with or without Ca) is beneficial (see public health implications below).
The inconclusive results from RCT of vitamin D supplementation increase the importance of evidence from cohort studies comparing baseline 25(OH)D levels with subsequent risk of fracture. At least ten of these cohort studies have been published, beginning with a very small study in 1990 (in which nine participants had fractures during 30-month follow-up) which is not included in the summary analysis since its sample of older adults was recruited from sheltered housing and not the general community(Reference Woo, Lau and Swaminathan36). The first community-based study was published in 1998(Reference Cummings, Browner and Bauer37), and the rest since 2005(Reference Gerdhem, Ringsberg and Obrant38–Reference Melhus, Snellman and Gedeborg45). The main outcomes examined have been hip and non-vertebral fractures (Table 1). The pooled relative risk is 1·34 (95 % CI 1·13, 1·59) comparing the lowest 25(OH)D quantile with the higher reference category in each study (Fig. 2), indicating a weak effect associated with low vitamin D status. The possibility of residual confounding remains as some studies did not control for BMI and physical activity, the two most important confounders (Table 1).
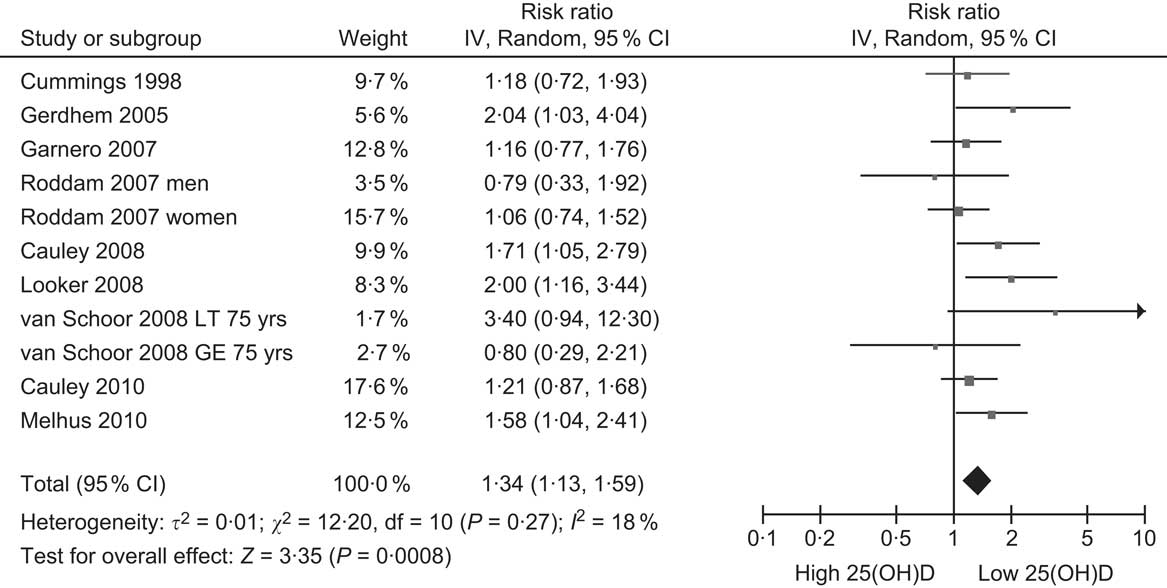
Fig. 2 Forest plot of relative risks of fracture associated with the lowest 25-hydroxyvitamin D (25(OH)D) category compared with the highest (or reference) in cohort studies
In summary, research on vitamin D and fractures appears to have lost momentum over the last 5 years, as indicated by the decline in publications during this period (Fig. 1). Conclusions from meta-analyses are inconsistent, because of low doses of vitamin D, often used in combination with Ca. However, the cohort studies indicate that people with low vitamin D levels are at increased risk of suffering fractures (Table 1), but these findings could be explained by other possible confounding factors. RCT giving higher doses of vitamin D than used previously are required to resolve the current uncertainty.
Cancer
A possible link between vitamin D and cancer first came from US ecological studies published around 1940 showing that a range of cancers were associated with sunlight exposure and latitude(Reference Peller and Stephenson46, Reference Apperly47).
However, the modern body of research on this topic is generally considered to have been stimulated by a later ecological study from US researchers who, unaware of the earlier studies, published a paper in 1980 showing an inverse association between latitude and colorectal cancer mortality in the USA(Reference Garland and Garland48). Since then, further ecological studies have shown inverse associations between solar radiation and cancers of the breast(Reference Garland, Garland and Gorham49), prostate(Reference Schwartz and Hulka50), ovary(Reference Lefkowitz and Garland51) and lymphoma(Reference Newton52), suggesting that vitamin D may protect against a wide range of cancers.
A substantial number of cohort studies, with baseline measures of 25(OH)D, have been carried out to test the hypotheses generated by the above ecological studies. These studies have been reviewed in a recent report by the International Agency for Research on Cancer (IARC)(53), since updated by the same authors(Reference Gandini, Boniol and Haukka54). Despite the extensive laboratory research showing the anticancer properties of vitamin D in prostate cells(Reference Moon, Fryer and Strange55), the current evidence does not support an association between vitamin D and prostate cancer risk. Eleven cohort studies of 25(OH)D and prostate cancer were published up until December 2009, with enough cases (n 3956) to detect a very small effect(Reference Gandini, Boniol and Haukka54). However, meta-analyses of these studies show no association between 25(OH)D and prostate cancer risk(53, Reference Gandini, Boniol and Haukka54, Reference Yin, Raum and Haug56). A further recent nested case–control study from Hawaii also found no association between baseline plasma 25(OH)D and prostate cancer risk(Reference Park, Cooney and Wilkens57), confirming the conclusions of the meta-analyses.
The evidence is stronger for breast cancer, with five cohort studies published up until December 2009 and the pooled relative risk being inverse but not statistically significant(Reference Gandini, Boniol and Haukka54, Reference Yin, Grandi and Raum58). For example, one meta-analysis calculated that a 50 nmol/l increase in 25(OH)D was associated with a relative risk of 0·92 (95 % CI 0·82, 1·04; P = 0·016)(Reference Yin, Grandi and Raum58), which suggests that if vitamin D is eventually confirmed to protect against breast cancer, the reduction in individual risk could be small. Since these meta-analyses, three further cohort studies of serum 25(OH)D and breast cancer have been published. One study from France reported a significant inverse association between baseline 25(OH)D and subsequent breast cancer risk(Reference Engel, Fagherazzi and Boutten59), a Swedish study reported a non-significant inverse association(Reference Almquist, Bondeson and Bondeson60), while further follow-up of mortality from the Third National Health and Nutrition Examination Survey (NHANES) cohort also has reported a non-significant inverse association(Reference Freedman, Looker and Abnet61). Thus, the overall evidence from cohort studies suggests there may be an inverse association between vitamin D status and subsequent risk of breast cancer, but further cohort studies are required to confirm this.
The cancer most strongly linked to vitamin D deficiency is colorectal cancer. To date, there are ten published cohort studies which have examined the association between baseline blood levels of 25(OH)D and incidence of colorectal cancer, beginning with the first report in 1989(Reference Garland, Comstock and Garland62) and the rest since the mid-1990s(Reference Freedman, Looker and Abnet61, Reference Braun, Helzlsouer and Hollis63–Reference Woolcott, Wilkens and Nomura70) (Table 2). Nine of these studies have been summarised in a meta-analysis, which found a significantly reduced risk of colorectal cancer with increasing 25(OH)D levels(Reference Gandini, Boniol and Haukka54). For a 25 nmol/l increase in 25(OH)D, colorectal cancer risk was decreased by 15 % (95 % CI 8, 21 %). An indication of the possible change in risk across the population range of 25(OH)D is shown in Fig. 3, with the pooled relative risk for people in the lowest 25(OH)D category being 1·59 compared with the lowest category. This suggests that the strength of the association between vitamin D and colorectal cancer is weak to moderate. The IARC report concluded that there was a significant inverse association between vitamin D status and colorectal cancer, but considered this to be ‘only limited evidence of a causal link due to confounding by other dietary or lifestyle factors’ (p. 305)(53). The main possible confounder is physical activity, since this is associated with both colorectal cancer(Reference Wolin, Yan and Colditz71) and 25(OH)D levels(Reference Scragg and Camargo72). Although physical activity was controlled for in several of the studies (Table 3), it is possible that errors in accurately measuring physical activity with questionnaires may have resulted in residual confounding and failure to control fully for its effects in multivariate analyses. Alternatively, it is also possible that any beneficial effect from physical activity could be from increased 25(OH)D levels resulting from activity. Only clinical trials of vitamin D supplementation will resolve this uncertainty.
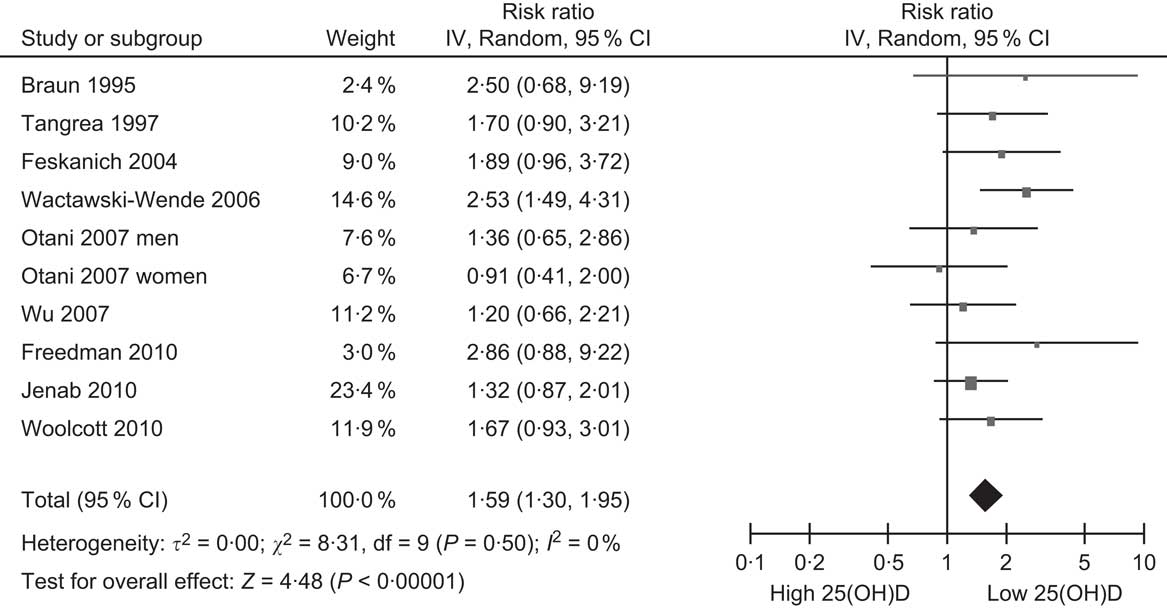
Fig. 3 Forest plot of relative risks of colorectal cancer associated with the lowest 25-hydroxyvitamin D (25(OH)D) category compared with the highest (or reference) in cohort studies (the study by Garland et al. (Reference Garland, Comstock and Garland62) is excluded because it did not report 95 % CI)
The main RCT carried out to date to see if vitamin D supplementation reduces incidence of colorectal cancer is the Women's Health Initiative. This study recruited 36 282 women aged 50–79 years into the vitamin D/calcium part of the wider study(Reference Wactawski-Wende, Kotchen and Anderson66). Women in the intervention arm of the study were given 10 μg of vitamin D and 1000 mg of Ca to take daily. At the end of the follow-up period (average of 7 years), there was no difference in the incidence of colorectal cancer between treatment and placebo arms. This negative finding could be due to some well-recognised limitations in the study design. These include: (i) too low a dose of vitamin D, which has been estimated to increase 25(OH)D levels only by about 7 nmol/l(Reference Heaney, Davies and Chen73); (ii) low compliance with only 70 % of participants taking the study capsules 50 % or more of the time; and (iii) contamination in the control group with many of them continuing to take vitamin D supplements during the follow-up period(Reference Newmark and Heaney74). One other RCT of vitamin D supplementation and cancer has been conducted(Reference Lappe, Travers-Gustafson and Davies75). In that study, 1179 women from Nebraska were given both vitamin D (27·5 μg/d) and Ca (1400–1500 mg/d) together, Ca only or placebo. After 4 years’ follow-up, women in both treatment arms had about half the risk of any type of cancer compared with those taking placebo, indicating a consistent beneficial effect for Ca. Thus, the role of vitamin D by itself is still unclear after this study and needs confirmation by other RCT.
The relationship between 25(OH)D levels and other rarer cancers has also been examined. In analyses pooling data from ten cohort studies, serum 25(OH)D was not associated with any of the following cancers: endometrial, oesophageal, gastric, kidney, non-Hodgkin lymphoma or ovarian; although risk of pancreatic cancer was found to be increased in people with 25(OH)D levels above 100 nmol/l(Reference Helzlsouer76). However, the latter finding was not confirmed in another US cohort study which reported a significant inverse association between predicted 25(OH)D level and risk of pancreatic cancer(Reference Bao, Ng and Wolpin77). Thus, the evidence on the association between vitamin D status and pancreatic cancer is inconsistent.
In summary, research on vitamin D and cancer has strong momentum, as indicated by the continuing increase in the annual number of publications during the last decade (Fig. 1). There is now a large body of laboratory work showing that active vitamin D (including calcitriol and its analogues) has anticancer properties by encouraging cell differentiation and proliferation of cancer cells from a range of tissues(Reference Gombart, Luong and Koeffler78). The cancer most strongly linked with low vitamin D status is colorectal cancer. However, this research has reached a tipping point, and RCT of vitamin D supplementation are now required to determine whether or not vitamin D protects against this cancer.
CVD
Research on vitamin D and CVD has increased more rapidly during the last decade than on other diseases (Fig. 1). This increase belies the major changes in scientific opinion about the role of vitamin D in CVD that has taken place over the last 40 years(Reference Scragg79). Until the 1970s, it was generally believed by researchers and clinicians that vitamin D was a cause of atherosclerosis and CHD(Reference Taussig80–Reference Kummerow84). These conclusions were based on animal studies which used pharmacological doses of vitamin D (125–250 μg/kg per d) in a cholesterol model to produce arteriosclerosis and on case reports of patients with arterial calcification and hypertension without controls to provide a reference point for vitamin D intake(Reference Scragg79). A major step was the development of the assays for measuring blood levels of 25(OH)D(Reference Hollis and Horst85), which showed that most vitamin D (>80 %) came from dermal production following sun exposure, not diet(Reference Haddad and Hahn11, Reference Poskitt, Cole and Lawson12).
Opinions about the role of vitamin D in CVD started to change (by 1980) with the publication of the hypothesis that UV radiation, through vitamin D formation, may protect against CVD(Reference Scragg86), which was subsequently expanded(Reference Scragg87); and the identification of a vitamin D receptor in rat heart(Reference Simpson88), along with animal studies showing that vitamin D affected cardiovascular function(Reference Weishaar and Simpson89). The first case–control studies to use the newly developed assay for 25(OH)D found, unexpectedly at the time, that myocardial infarction cases had similar or lower 25(OH)D levels than controls(Reference Schmidt-Gayk, Goossen and Lendle90–Reference Vik, Try and Thelle92). The initial test of the hypothesis that vitamin D may protect against CVD was a population-based case–control study from New Zealand, published in 1990, which found a significant inverse association between 25(OH)D levels and risk of myocardial infarction(Reference Scragg, Jackson and Holdaway93). However, because of the case–control design with collection of blood samples after the onset of disease, uncertainty remained as to whether or not 25(OH)D levels in cases had been affected by the disease process.
The first cohort study comparing baseline 25(OH)D levels and risk of subsequent CVD was not published until 2005. That study of elderly people in Finland showed a significant inverse association between baseline 25(OH)D levels and subsequent risk of acute myocardial infarction, but not of stroke (Table 3)(Reference Marniemi, Alanen and Impivaara94). The study was initially unnoticed, possibly because vitamin D was one of many nutrients examined. The publication that drew the notice of researchers was from the Framingham Offspring Study which found that participants with 25(OH)D levels <10 μg/ml (25 nmol/l) had nearly double the risk of CVD during follow-up compared with those ≥15 μg/ml (37·5 nmol/l)(Reference Wang, Pencina and Booth95). Since then, there has been a rush of publications from cohort studies of community-based samples, with eleven other studies reporting on the association between 25(OH)D levels and risk of CVD (Table 3)(Reference Giovannucci, Liu and Hollis96–Reference Semba, Houston and Bandinelli106). Although not all of these studies have reported significant inverse associations, when combined, overall they show that participants in the lowest 25(OH)D category have a 35 % increased risk of CVD compared with the reference category, after adjusting for covariates including (in most studies) obesity and physical activity (Fig. 4). Still, this small increase in risk indicates that the association is a weak one, and residual confounding remains a possible explanation for it.
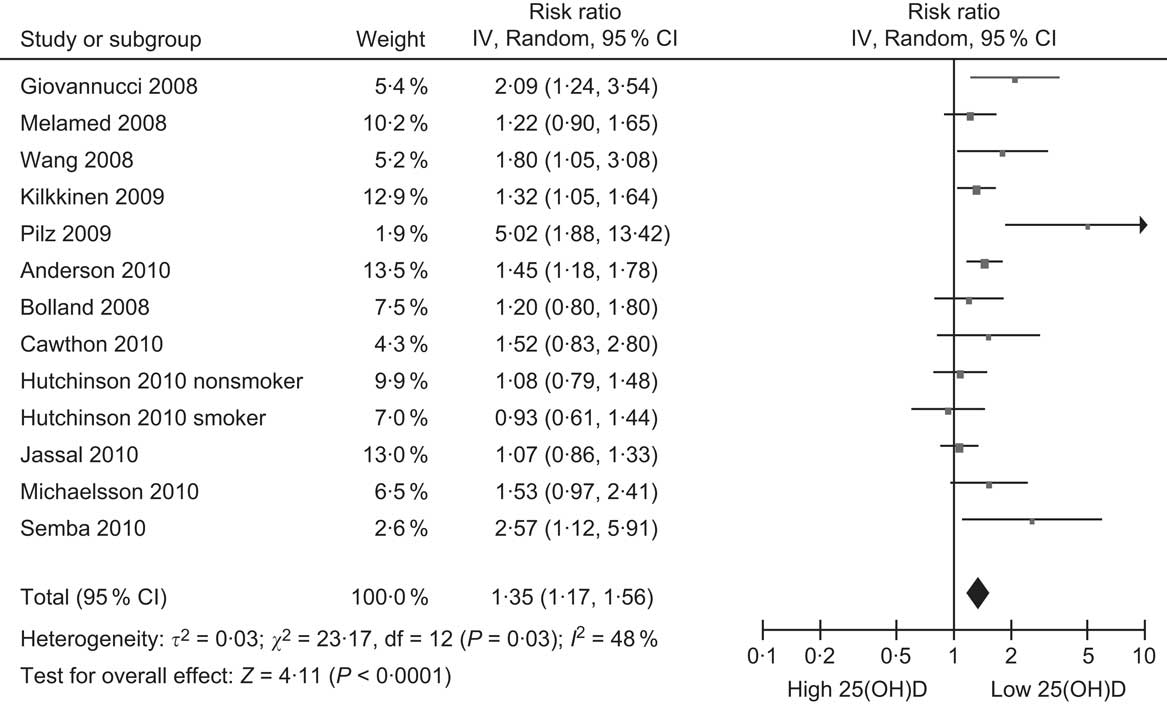
Fig. 4 Forest plot of relative risks of CVD associated with the lowest 25-hydroxyvitamin D (25(OH)D) category compared with the highest (or reference) in cohort studies (the study by Marniemi et al. (Reference Marniemi, Alanen and Impivaara94) is excluded because it reported relative risks separately for myocardial infarction and stroke)
Only one RCT with sufficient power to detect any beneficial effect on CVD incidence has been carried out. This is the Women's Health Initiative RCT, which randomised over 36 000 women aged 50–79 years to taking daily doses of vitamin D (10 μg) and Ca (1000 mg) or placebo (as reported above for colorectal cancer). There was no difference between treatment and placebo groups in the risk of CVD during the 7-year follow-up period(Reference Hsia, Heiss and Ren107). The lack of a treatment effect is attributed to the limitations of this trial(Reference Michos and Blumenthal108), as outlined above for colorectal cancer.
Research has also examined the association that vitamin D status has with cardiovascular risk factors, particularly blood pressure and type 2 diabetes. For blood pressure, there have been numerous cross-sectional studies and at least four cohort studies which have compared baseline 25(OH)D levels with subsequent risk of hypertension(Reference Geleijnse109, Reference Burgaz, Orsini and Larsson110), with a 27 % reduction in risk of hypertension for participants in the highest vitamin D category compared with the lowest(Reference Burgaz, Orsini and Larsson110). However, RCT of vitamin D supplementation and blood pressure have produced conflicting results, with most long-term studies showing no beneficial effect(Reference Geleijnse109). It is possible that vitamin D may affect arterial function through different mechanisms than blood pressure, since intimal medial thickening of carotid arteries is inversely associated with 25(OH)D(Reference Targher, Bertolini and Padovani111), although vitamin D supplementation has been found to have inconsistent effects on endothelial function(Reference Sugden, Davies and Witham112, Reference Witham, Dove and Dryburgh113).
With regard to type 2 diabetes, there have also been numerous cross-sectional studies showing inverse associations between blood 25(OH)D levels and risk of diabetes(Reference Pittas, Lau and Hu114), and at least five cohort studies showing that baseline 25(OH)D levels predict diabetes or other glycaemic measures(Reference Mattila, Knekt and Mannisto115–Reference Liu, Meigs and Pittas119). Another cohort study has reported that people with increased sun exposure have a reduced risk of diabetes(Reference Lindqvist, Olsson and Landin-Olsson120). However, the few RCT to date have shown inconsistent results(Reference Jorde and Figenschau121–Reference Alvarez and Ashraf123).
In summary, vitamin D research over the last 10 years has increased more rapidly for CVD than any other disease group (Fig. 1). A substantial number of community-sampled cohort studies have been carried out which collectively show that low baseline blood 25(OH)D levels predict increased risk of CVD (Fig. 4). Substantial clinical and laboratory research has identified a range of mechanisms by which vitamin D may affect risk of CVD, including alterations in immune function, cardiac size and function, insulin resistance and arterial function(Reference Reddy Vanga, Good and Howard124). However, the association between vitamin D and CVD is weak and, as for colorectal cancer, large RCT are required to confirm the findings from the observational studies.
All-cause mortality
The accumulating research on vitamin D and CVD inevitably led to an interest in the possible association between vitamin D and all-cause mortality, given that CVD is the leading cause of mortality in developed countries. Research on this topic has been stimulated greatly by a recent meta-analysis of RCT originally undertaken to determine the effect of vitamin D supplementation on risk of fractures (summarised above). This meta-analysis found that participants in these trials given vitamin D had a 7 % decrease in all-cause mortality compared with those receiving placebos(Reference Autier and Gandini125). The weighted dose of vitamin D in these trials was only 528 IU/d, suggesting that vitamin D may decrease mortality by much greater amounts if given in higher doses. This analysis has stimulated other researchers of cohort studies to include all-cause mortality as an outcome, along with CVD (Table 3). Surprisingly, the first cohort study to report on the association between baseline 25(OH)D levels and subsequent risk of death, which was from the Netherlands, was missed in the subsequent rush of papers on this topic, perhaps because its focus was on predicting admission to nursing homes rather than death(Reference Visser, Deeg and Puts126). However, since then, eleven other cohort studies have reported on this association (Table 3)(Reference Melamed, Michos and Post97, Reference Pilz, Dobnig and Nijpels99–Reference Hutchinson, Grimnes and Joakimsen103, Reference Michaelsson, Baron and Snellman105, Reference Semba, Houston and Bandinelli106, Reference Jia, Aucott and McNeill127–Reference Szulc, Claustrat and Delmas129). The summary relative risk from these studies is 1·42, when comparing the lowest 25(OH)D category with the reference (Fig. 5), which indicates a weak association across the range of 25(OH)D concentrations in the general population. Although a small effect, a reduction of this amount in all-cause mortality would be substantial, so it is potentially of great health significance. However, large RCT are required to confirm if vitamin D supplementation by itself reduces all-cause mortality since another meta-analysis has concluded that vitamin D is beneficial only when combined with Ca(Reference Avenell, Gillespie and Gillespie23).
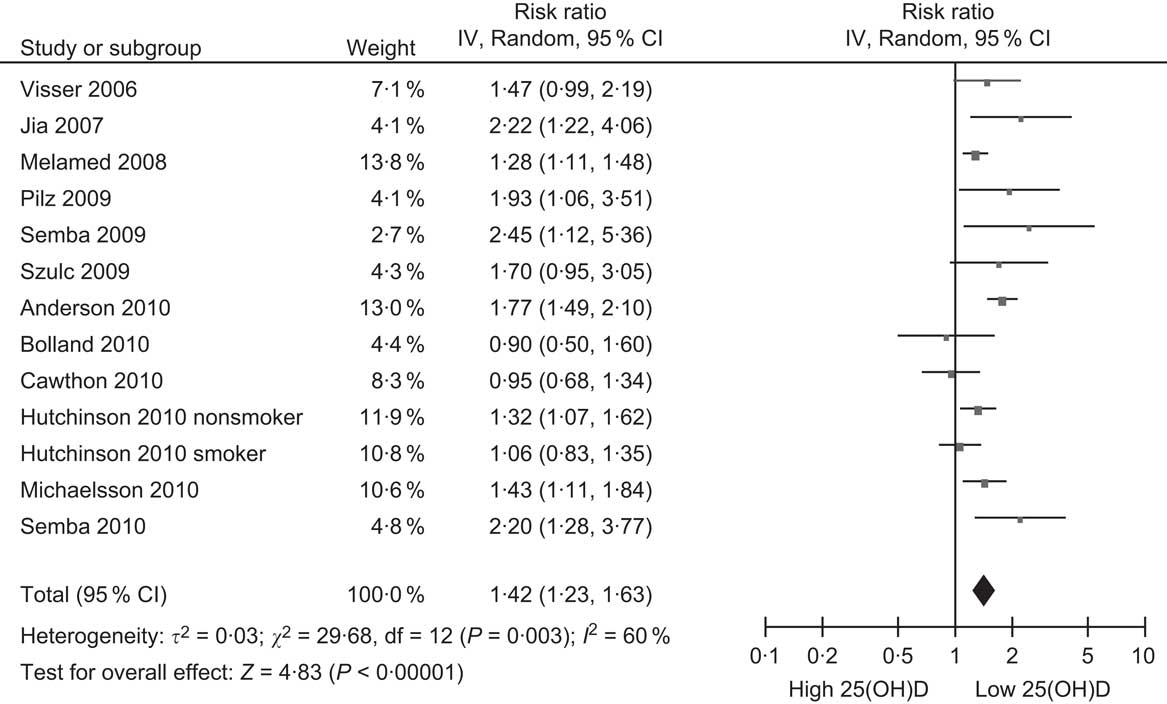
Fig. 5 Forest plot of relative risks of all-cause mortality associated with the lowest 25-hydroxyvitamin D (25(OH)D) category compared with the highest (or reference) in cohort studies
Infection and immune function
The final disease group considered in the present overview is infection and the effects of vitamin D on immune function. Research on the beneficial effects of vitamin D against infection extends back to the beginning of the 20th century when Nils Finsen, a physician from Copenhagen, was awarded the Nobel Prize for the heliotherapy he developed using the eponymous lamp to cure lupus vulgaris, a disfiguring skin disease caused by tuberculosis (TB) which typically affects the face(130). The success of his treatment eventually led to the establishment of sanatoria for TB patients where a key part of the treatment was exposure to sunlight(Reference Rollier131). Up until the early 1950s, patients with TB were being increasingly treated with vitamin D(Reference Dowling and Prosser Thomas132). However, the introduction of antibiotics in the 1950s, with their greater effectiveness, soon resulted in the abandonment of vitamin D as a therapeutic agent against TB.
Immune function can be split broadly into two components: innate and acquired. The key agents of the innate immune system are antimicrobial peptides, which are the immediate, non-specific, first line of defence for plants and animals against infectious organisms(Reference Boman133). The main antimicrobial peptide in man is cathelicidin. An influential paper published in 2006 showed that vitamin D increases synthesis of cathelicidin(Reference Liu, Stenger and Li134), and it is through this mechanism that vitamin D is thought to protect against TB and other infectious diseases(Reference Gombart135, Reference Bartley136). Low plasma levels of cathelicidin have recently been shown to predict increased mortality from infectious disease in renal dialysis patients(Reference Gombart, Bhan and Borregaard137). A meta-analysis has shown that TB cases have lower 25(OH)D levels than controls(Reference Nnoaham and Clarke138), and vitamin D has been used in recent RCT with mixed results(Reference Martineau, Honecker and Wilkinson139, Reference Martineau, Timms and Bothamley140). There is also increasing evidence of a link between vitamin D status and viral respiratory tract infections. Observational studies mostly show lower 25(OH)D levels in cases compared with controls, and four out of five RCT have shown that vitamin D supplementation reduces incidence of respiratory tract infection or influenza(Reference Beard, Bearden and Striker141).
Vitamin D also affects acquired immune function and risk of related autoimmune diseases, such as multiple sclerosis and type 1 diabetes. The occurrence of multiple sclerosis increases with latitude(Reference McLeod, Hammond and Hallpike142) and evidence from observational studies shows that decreased sun exposure and 25(OH)D levels are risk factors for this disease(Reference van der Mei, Ponsonby and Dwyer143–Reference Lucas, Ponsonby and Dear145). For type 1 diabetes, animal studies over 30 years ago identified a pancreatic receptor to vitamin D(Reference Christakos, Friedlander and Frandsen146) and showed that vitamin D deficiency decreased insulin secretion by the pancreas(Reference Norman, Frankel and Heldt147). A meta-analysis of observational studies in man has confirmed these findings, with a history of taking vitamin D supplements during childhood being associated with a 29 % decreased risk of type 1 diabetes compared with children who did not take vitamin D(Reference Zipitis and Akobeng148).
In summary, research on vitamin D and immune function is increasing (Fig. 1). The evidence indicates that vitamin D may protect against a wide range of infections including TB and respiratory infections. RCT that are currently underway should provide a clear picture within the next 5–10 years as to whether vitamin D is effective against these diseases.
Institute of Medicine report
The IOM in the USA released its report on the recommended dietary intakes of Ca and vitamin D at the end of November 2010(4). It is a substantial document, based largely on two commissioned systematic reviews. The first review, by researchers in Ottawa, summarised the literature on vitamin D and bone health published up to mid-2006, with a focus on RCT(Reference Cranney, Horsley and O'Donnell149). The second review, by researchers in Boston, was of publications up to April 2009(Reference Chung, Balk and Brendel150). It updated the bone data published since the Ottawa review, but also reviewed many other health outcomes. It focused on RCT and cohort studies, and excluded cross-sectional and case–control studies. The IOM report mainly relied on these two reviews, but also considered data from other study designs including observational studies (e.g. case–control and cross-sectional) in man and animal studies of biological mechanisms. It assessed the evidence on vitamin D for a wide range of outcomes (Table 4.1 of the IOM report), which surprisingly did not include all-cause mortality. Its main conclusions with regard to vitamin D were that: (i) vitamin D status was related only to bone health; and (ii) 25(OH)D levels of 50 nmol/l represent vitamin D sufficiency because above this level there was no consistent evidence of increased health benefit. On this basis, the IOM decided to marginally increase the dietary reference intake (from the previous recommendations in their 1997 report) to 15 μg/d for adults up to 70 years of age and 20 μg/d above this age. The smallness of this increase has surprised many vitamin D researchers and clinicians, since these daily intakes will increase 25(OH)D levels by only 5–20 nmol/l depending on a person's starting 25(OH)D level(Reference Garland, French and Baggerly8).
As with any report of this magnitude, the evidence on which its conclusions are based will rapidly become out of date; this is already happening with the IOM report. For example, seven of the nine cohort studies of fractures in Table 1, all published from 2007(Reference Garnero, Munoz and Sornay-Rendu39–Reference Melhus, Snellman and Gedeborg45), are not mentioned in the IOM report or the systematic reviews on which it is based; while for colorectal cancer, two out of the ten cohort studies in Table 2 were not included(Reference Freedman, Looker and Abnet61, Reference Woolcott, Wilkens and Nomura70), one of which(Reference Freedman, Looker and Chang151) was an update of the third NHANES included in the report.
However, it is in the areas of CVD and all-cause mortality, where publications are more recent, that the omissions are greatest. In the section on CVD, there is no mention in the IOM report about nine of the cohort studies of CVD in Table 3(Reference Kilkkinen, Knekt and Aro98–Reference Semba, Houston and Bandinelli106), all published from 2009, although the study by Bolland et al.(Reference Bolland, Bacon and Horne101) is described elsewhere in the report. Instead, the IOM report focuses on the papers from the Framingham Offspring Study, which it describes as having a U-shaped association between CVD risk and 25(OH)D above 75 nmol/l(Reference Wang, Pencina and Booth95), and the initial mortality report from NHANES which it also described as having a U-shaped dose–response relationship(Reference Melamed, Michos and Post97); but made no mention in the discussion of chapter 4 on health outcomes of a further analysis of the NHANES data of people aged ≥65 years which found an inverse linear association across the population distribution of 25(OH)D rather than a U-shaped relationship(Reference Ginde, Scragg and Schwartz152). The possible U-shaped dose–response relationship is one of the rationales used by the IOM for keeping the cut-off point for vitamin D sufficiency at a 25(OH)D concentration of 50 nmol/l. Of the cohort studies on CVD not cited in the IOM report which had more than two 25(OH)D comparison groups, two described a U-shaped association in all(Reference Michaelsson, Baron and Snellman105) or some (i.e. non-smokers)(Reference Hutchinson, Grimnes and Joakimsen103) of their participants and four showed evidence of an inverse linear association(Reference Kilkkinen, Knekt and Aro98, Reference Anderson, May and Horne100, Reference Cawthon, Parimi and Barrett-Connor102, Reference Semba, Houston and Bandinelli106). If the latter are combined with the other publication mentioned by the IOM from the study of US male health professionals which showed a significant dose–response association(Reference Giovannucci, Liu and Hollis96), then the emphasis by the IOM about a likely U-shaped dose–response relationship between 25(OH)D and CVD, and their conclusion that the 25(OH)D evidence does not show a relationship with risk for developing CVD, do not look convincing.
Death trumps all other outcomes, so even more surprising is the absence of all-cause mortality from the list of health outcomes in Table 4·1 of the IOM report, even though this outcome was examined in one of the systematic reviews used by the report(Reference Chung, Balk and Brendel150). The meta-analysis of RCT of vitamin D supplementation (with or without Ca) is not even listed in the references of the IOM report, despite being published in 2007(Reference Autier and Gandini125). Seven of the twelve cohort studies in Table 3 which have published data on the association between 25(OH)D and all-cause mortality are not referenced in the IOM report(Reference Pilz, Dobnig and Nijpels99, Reference Anderson, May and Horne100, Reference Cawthon, Parimi and Barrett-Connor102, Reference Hutchinson, Grimnes and Joakimsen103, Reference Michaelsson, Baron and Snellman105, Reference Semba, Houston and Bandinelli106, Reference Szulc, Claustrat and Delmas129). This omission is all the more surprising when the IOM report includes many references from 2010, which suggests that there was selective reporting of publications from the two years (2009 and 2010) preceding the release of the report.
Despite the above limitations, the additional publications from cohort studies since 2009, which have added greatly to the body of evidence, have not diminished but increased the need for evidence from large RCT to determine with greater certainty whether vitamin D protects against the diseases covered in the present overview. By increasing the dietary reference intake only up to 20 μg/d (for the oldest age groups) the IOM has provided a window of opportunity for researchers to undertake RCT for disease outcomes. If the daily dietary reference intake had been increased up to 50 μg or higher, it would have been ethically difficult for researchers to do RCT as they would have been obligated to give participants in the control arm this high dose. The resulting contamination in the control arm would have made it difficult to detect any beneficial effect from vitamin D. At least two large RCT using higher doses of vitamin D have started. These are: (i) the US VITAL study (www.vitalstudy.org), which is recruiting 20 000 older adults to determine if 50 μg vitamin D3/d prevents cancer and CVD (ClinicalTrials.gov identifier: NCT01169259); and (ii) the ViDA study from New Zealand, which is recruiting 5100 older adults to determine if 2·5 mg vitamin D3/month prevents CVD, respiratory disease (including infections) and non-vertebral fractures (www.ANZCTR.org.au registration number: ACTRN12611000402943).
Public health implications
Why do we need RCT to prove the benefit of vitamin D if the cohort data look so convincing? First, the recent evidence for other micronutrients, such as vitamins A, B, C and E, has shown that results from observational studies, which generally reported inverse associations between nutrient status and risk of cancer and CVD disease, have not been confirmed by RCT; in fact, supplementation increases mortality(Reference Byers1, Reference Bjelakovic, Nikolova and Gluud2). Hence the need for caution with vitamin D and the need to show that it is both safe and effective; although vitamin D may be different from other micronutrients because it is a hormone(Reference Morabia and Costanza153). Second, the ultimate goal of vitamin D research, if vitamin D is truly beneficial, must be for state-funded programmes to increase vitamin D levels in the general population through the available strategies, such as increased (but safe) sun exposure, fortification and supplementation. If the state is to invest in such programmes, there will be an opportunity cost (from less money to spend on other programmes) and it needs evidence from RCT and cost-effectiveness analyses to show that any benefit from investment in vitamin D programmes is equivalent to or higher than that for other publicly funded interventions. In the absence of state-funded programmes, health inequalities will increase if vitamin D is beneficial, since poorer sections of the community, which typically have the lowest vitamin D levels, are likely to have the least capacity to increase their vitamin D levels.
Assuming that RCT show benefit, the strategies used in future public health programmes will be influenced by some of the disputed evidence described above. For example, the uncertainty about whether vitamin D is associated linearly or in a U-shaped pattern with disease risk will determine whether population or high-risk approaches to prevention are used(Reference Rose154). If the association between vitamin D status and risk of disease were inversely linear, then programmes targeting the general population would be required. However, if the association is U-shaped, then high-risk strategies which identify and target people with low vitamin D levels would be preferred, since people with high levels would not gain from having even higher vitamin D levels.
Another point of contention, which will shape the type of interventions, is whether vitamin D is beneficial by itself, or whether it needs to be combined with Ca. Ca is a much more expensive supplement than vitamin D to use in public health campaigns since it needs to be taken on a daily basis because of its water solubility. As well, there are safety issues with Ca(Reference Bolland, Avenell and Baron155) that were downplayed in the IOM report. In contrast, vitamin D is fat soluble and can be taken less often (e.g. monthly)(Reference Ish-Shalom, Segal and Salganik156). This greatly decreases the cost, and respondent burden, for running population-wide supplementation programmes.
Conclusions
Cohort studies show that baseline 25(OH)D levels predict increased risk of fractures, colorectal cancer, CVD and all-cause mortality. However, these associations are weak, with relative risks comparing the lowest 25(OH)D category with the highest (or reference) being in the range of 1·3 to 1·6. While these could be underestimates of the true effect (for reasons discussed in the Methods), it is also possible they are caused by confounding from other variables such as obesity and physical activity. RCT using vitamin D doses ≥50 μg/d are required to determine whether vitamin D protects against these diseases. The evidence in the IOM report is now out of date for the above outcomes. However, its recommendation for daily vitamin D intakes up to 20 μg provides a window for researchers to undertake higher-dose RCT. These are now starting and should provide definitive answers about the health benefits of vitamin D during the coming decade.
Acknowledgements
Funding was provided by the Health Research Council of New Zealand and the Accident Compensation Commission. No conflicts of interest declared.












