Breast cancer is a leading cause of cancer mortality worldwide and the most common form of cancer affecting women( Reference Parkin, Bray and Ferlay 1 ). In 2012, approximately 1·7 million women were diagnosed with breast cancer, with 522 000 related deaths – an increase in breast cancer incidence and related mortality by nearly 18 % from 2008( Reference Ferlay, Soerjomataram and Dikshit 2 ). In Japan, the incidence of breast cancer has continuously and rapidly increased over the last three decades, and breast cancer is currently the most common cancer affecting females, as in Western countries( Reference Matsuda, Marugame and Kamo 3 ). Diet is a modifiable risk factor for breast cancer, and several single foods and nutrients have been examined in association with breast cancer risk, including vegetables, fibre and vitamins( Reference Gandini, Merzenich and Robertson 4 – Reference Michels, Mohllajee and Roset-Bahmanyar 6 ). However, results from studies on individual foods and nutrients may be inconsistent because these cannot account for the complex interactions that occur between the various nutrients and other components of different foods( Reference Edefonti, Randi and La Vecchia 7 , Reference Velie, Schairer and Flood 8 ). Furthermore, dietary patterns in Asian countries differ from those in Western countries: the traditional Japanese diet, for example, is composed primarily of steamed rice, miso soup, various vegetables, fermented foods with salt and fish. Thus, many researchers have recently focused on dietary pattern analysis, a statistical method that describes the overall diet. Several previous studies investigating dietary patterns in relation to breast cancer risk have shown an inverse association with the prudent/healthy dietary pattern, which usually includes high intake of vegetables, fruits, whole grains and fish( Reference Adebamowo, Hu and Cho 9 – Reference Wu, Yu and Tseng 13 ), and a positive association with the western/unhealthy pattern, which is generally characterised by high intake of red meat, refined grains, potatoes and fat( Reference Cui, Dai and Tseng 14 – Reference Fung, Hu and Holmes 17 ). Given that dietary patterns are likely to vary among populations because of geographic characteristics and cultural differences in food habits, preferences and availability( Reference Hu 18 ), these reports are of limited value for the prevention and management of breast cancer in the Japanese population. Moreover, information from Japanese populations is scant: one study in Japanese women reported that the prudent dietary pattern decreased the risk of breast cancer, but this was carried out using a case–control design( Reference Hirose, Matsuo and Iwata 10 ), and no prospective study among Japanese women has yet been conducted.
In this study, using data from the Japan Public Health Center-based Prospective Study (JPHC Study), we evaluated the relationship between dietary pattern and risk of breast cancer among Japanese women. Further, we identified associations by subtype and hormone receptor status using principal component factor analysis.
Methods
Study population
The JPHC Study was launched in 1990 for Cohort I and in 1993 for Cohort II. Details of the study design have been described previously( Reference Tsugane and Sawada 19 ). This study was approved by the Institutional Review Board of the National Cancer Center of Japan and the University of Tokyo. A total of 140 420 subjects were invited to participate in the baseline survey (1990–1994), of whom 71 698 were women. Subjects registered at one public healthcare centre area (n 4178) were excluded because information on cancer incidence was not available. Cohort participants responded to a self-administered questionnaire at baseline from 1990 to 1994. A 5-year follow-up survey was conducted from 1995 to 1998. Compared with the original baseline survey, the follow-up provided greater detail on the frequency of food intake, and was therefore selected as baseline for the present study. After exclusion of subjects who were ineligible (non-Japanese ethnicity, late report of migration occurring before the start of the study or incorrect birth data), deceased or had moved out of a study area, 62 787 women were eligible for participation. Of these, 52 484 women responded to the 5-year follow-up survey (response rate=84 %). We further excluded participants with a self-reported history of cancer occurring before the start of the follow-up (n 1861), and those with extreme total energy intake (±3 sd) at the 5-year follow-up survey (n 581). Consequently, 49 552 women were ultimately enrolled to assess the association between dietary pattern and breast cancer risk.
Assessment of dietary pattern
Dietary assessment was performed using a validated self-reported FFQ. The 5-year follow-up survey enquired about the consumption of 147 food and beverage items over the previous year( Reference Sasaki, Kobayashi and Ishihara 20 ). The frequency of most food items was divided into nine categories (never, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, 1 time/d, 2–3 times/d, 4–6 times/d, and ≥7 times/d). Slightly different categories were used for beverage intake. A standard portion size was specified for each food item, and respondents were asked to choose their usual portion size from three options (less than half, standard or >1·5 times). Daily food intake was calculated by multiplying daily consumption frequency by the individual’s usual portion size. The validity and reproducibility of the FFQ have both been described previously( Reference Ishihara, Sobue and Yamamoto 21 – Reference Sasaki, Ishihara and Tsugane 23 ).
Details regarding the identification of dietary patterns have been described elsewhere( Reference Nanri, Shimazu and Ishihara 24 ). In brief, we used 134 food and beverage items from the FFQ to derive dietary patterns. Some foods or food groups that were similar in nutritional content or culinary use were combined, making a total of forty-eight food groups. We performed principal component analysis based on log-transformed intakes of these forty-eight food groups. We determined factors by eigenvalues of >1·75 and a scree plot and interpretability of the derived factors. The factors were then rotated by orthogonal transformation (varimax rotation) to increase interpretability while maintaining uncorrelated factors. Dietary patterns were named according to food items that had the highest weight in each of the three factors. The factor scores for each dietary pattern were calculated for each participant by summing the intake of food items weighted by their factor loadings. Scores were energy adjusted using the residual method. The validity and reproducibility of the identified dietary patterns were determined to be acceptable( Reference Nanri, Shimazu and Ishihara 24 ). Regarding reproducibility, Spearman’s correlation coefficients between the two FFQ ranged from 0·55 for the prudent pattern to 0·71 for the westernised pattern in women( Reference Nanri, Shimazu and Ishihara 24 ). Regarding validity, the corresponding values between dietary records and the FFQ ranged from 0·36 for the prudent pattern to 0·63 for the traditional pattern in women( Reference Nanri, Shimazu and Ishihara 24 ).
Ascertainment of breast cancer cases and follow-up of the cohort
Breast cancer cases were identified through active patient notification from major local hospitals in the study area and data linkage with population-based cancer registries, with permission from the local governments responsible for the registries. Breast cancer cases were defined with reference to the Third Edition of the International Classification of Diseases for Oncology as codes C500-509. Information on oestrogen receptor (ER) and progesterone receptor (PR) status was evaluated by either immunohistochemical assay or ELISA. The cut-off points for a positive status for ER and PR were defined by clinical estimation for medical treatment or were specified by the assay method.
Subjects were enrolled to the study on the administration date of the 5-year follow-up survey, and contributed person-time from enrolment until the date of diagnosis with breast cancer, date of death, date of moving away from the study area or the end of follow-up (31 December 2012), whichever occurred first.
Statistical analysis
We used multivariable Cox proportional hazards regression models to examine the hazard ratios (HR) and 95 % CI for breast cancer risk across the quintile categories of each dietary pattern score, taking the lowest quintile category as reference. The basic model was adjusted for age (<50, 50–54·9, 55–59·9, 60–64·9, 65–69·9, ≥70 years) and public healthcare centre area, whereas the multi-variable model was further adjusted for log-transformed energy intake (continuous), BMI (<22·5, 22·5–24·9, 25–27·4, ≥27·5 kg/m2), smoking status (never, past, current), leisure-time physical activity (<1 d/month, 1–3 d/month, ≥1 d/week), total physical activity (metabolic equivalent task-h/d), age at menarche (≤13, 14, 15, ≥16 years, missing), parity (nulliparous, 1, 2–3, ≥4), age at first birth (nulliparous,<26, ≥26 years, missing), menopause status (premenopause, age at menopause <51, ≥51 years) and use of exogenous female hormones (never, ever). Trend associations were assessed by assigning ordinal numbers (0–4) to quintile categories of each dietary pattern. We performed an analysis stratified by menopausal status. We focused on menopausal status obtained at the time of diagnosis (the censored date). As no previous study had direct information on menopausal status at the time of diagnosis for breast cancer, we used 51 years as a proxy cut-off for menopausal age at the 5-year follow-up survey, the time at which approximately 50 % of the women had become postmenopausal( Reference Wada, Nagata and Tamakoshi 25 ). Postmenopausal breast cancer was defined in those who reported being postmenopausal at the 5-year follow-up survey and/or were 51 years or older at the time of diagnosis. All other breast cancers were defined as premenopausal. For non-cases of breast cancer, when participants were 51 years or older and/or reported being postmenopausal at baseline, the years of observation were considered as postmenopausal period. When women who reported no to being postmenopausal at baseline were younger than 51 years of age at the censored date, the years of observation were considered as premenopausal period. We computed P interaction values using a likelihood-ratio test to compare Cox proportional hazards models with and without cross-product terms for menopausal status and each dietary pattern category in analyses stratified by menopausal status. The associations were also evaluated according to ER and PR status, as well as for combinations of them. All P values presented are two-tailed and were considered to be statistically significant when P<0·05. All statistical analyses were conducted using SAS version 9.3 software (SAS Institute Inc.).
Results
Three main dietary patterns were derived from 49 552 Japanese women from the JPHC Study (Table 1). Patterns were named according to the food groups that had high loadings or the characteristics of the food group that composed the dietary pattern. The prudent dietary pattern was characterised by high consumption of vegetables, fruits, soya products, potatoes, seaweed, mushroom, and fish; the westernised dietary pattern was correlated with high intake of bread, meat, processed meats, dairy products, soup, coffee, soft drinks, black tea, sauces, mayonnaise and dressing; and the traditional Japanese dietary pattern was characterised by high intake of salmon, seafood other than fish, oily fish, lean fish, salty fish, chicken and pickles. These three dietary patterns account for 29·9 % of the variability in the standardised dietary intake, at 20·7, 5·5 and 3·7 % for the prudent, westernised and traditional Japanese diets, respectively.
Table 1 Factor-loadingFootnote * matrix for major dietary patterns identified by principal component analysis (n 49 552)

* Factor loading scores less than −0·14 and +0·14 are not shown.
Subject characteristics by quintile category of the three dietary patterns are presented in Table 2. Women in the highest quintile of the prudent dietary pattern score were more likely to be older, shorter, more active, have low total energy intake, low tobacco use, low alcohol use, earlier menarche, later first delivery and later menopause compared with women in the lowest quintile of the prudent dietary pattern. Subjects with a higher score for the westernised dietary pattern were more likely to be younger, taller, have higher use of tobacco, higher use of alcohol, earlier menarche, later first delivery, less parity, earlier menopause and use of exogenous female hormones. Subjects with a higher score for the traditional Japanese dietary pattern tended to be younger, have low BMI, low total energy intake, high alcohol consumption, earlier menarche, earlier first delivery and less parity.
Table 2 Subject characteristics according to quintiles (Q) of dietary pattern score at the 5-year follow-up study (Mean values and standard deviations)

MET, metabolic equivalent task.
* χ 2 Test for ordinal qualitative variables and linear regression for continuous variables.
During 725 534 person-years of follow-up (average follow-up: 14·6 years) for 49 552 women, a total of 718 cases of breast cancer were newly diagnosed and included in the analyses. Information on ER and PR status was available for 356 cases (50 % of total breast cancer), of which 155 were oestrogen receptor-positive (ER+)/progesterone receptor-positive (PR+), fifty-eight were ER+/progesterone receptor-negative (ER-), eighty-five were oestrogen receptor-negative (ER-)/PR- and eleven were ER-/PR+ (310 cases had known ER/PR status). HR of overall breast cancer according to quintile category of each dietary pattern score are shown in Table 3. The westernised dietary pattern was associated with an overall increase in breast cancer risk (HR 1·32; 95 % CI 1·03, 1·70; P trend=0·04). We conducted additional analyses to identify the association between extreme intake of a westernised diet and breast cancer risk among women in the highest quintile category (Q5) of the westernised dietary pattern. Q5 of the westernised dietary pattern was further divided into additional extreme quintile categories labelled Q5_1st, Q5_2nd, Q5_3rd, Q5_4th and Q5_5th. Women categorised in Q5_5th of the westernised dietary pattern were thus in the top 4 % of total subjects. The results showed that they had a 63 % increase in the risk of breast cancer (HR 1·83; 95 % CI 1·25, 2·68; P trend=0·01) compared with those in the original lowest Q1 (Tables 4).
Table 3 Breast cancer risk according to quintiles (Q) of dietary pattern score (Hazard ratios (HR) and 95 % confidence intervals)

Ref., referent values.
* Based on a Cox proportional hazards model, with ordinal numbers 0–4 assigned to the quintile categories of each dietary pattern.
† Multivariable adjusted: multivariable Cox proportional hazards model were adjusted for age (<50, 50–54·9, 55–59·9, 60–64·9, 65–69·9, ≥70 years), public healthcare centre area (ten), log-transformed energy intake (continuous), BMI (<22·5, 22·5–24·9, 25–27·4, ≥27·5 kg/m2), smoking status (never, past, current), leisure-time physical activity (<1 d/month, 1–3 d/month, ≥1 d/week), total physical activity (<30, ≥30 metabolic equivalent task-h/d, missing), age at menarche (≤13, 14, 15, ≥16 years, missing), parity (nulliparous, 1, 2–3, ≥4), age at first birth (nulliparous, <26, ≥26 years, missing), menopause status (premenopause, age at menopause <51, ≥51 years) and use of exogenous female hormones (never, ever).
Table 4 Breast cancer risk according to quintiles (Q) among the highest quintile group (Q5) (n 9 910, breast cancer cases=166) (Hazard ratios (HR) and 95 % confidence intervals)
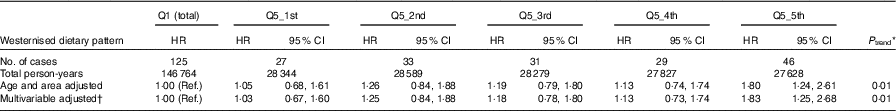
Ref., referent values.
* Based on a Cox proportional hazards model, with ordinal numbers 0–4 assigned to the quintile categories of each dietary pattern.
† Multivariable adjusted: multivariable Cox proportional hazards model was adjusted for age (<50, 50–54·9, 55–59·9, 60–64·9, 65–69·9, ≥70 years), public health centre area (ten), log-transformed energy intake (continuous), BMI (<22·5, 22·5–24·9, 25–27·4, ≥27·5 kg/m2), smoking status (never, past, current), leisure-time physical activity (<1 d/month, 1–3 d/month, ≥1 d/week), total physical activity (<30, ≥30 metabolic equivalent task-h/d, missing), age at menarche (≤13, 14, 15, ≥16 years, missing), parity (nulliparous, 1, 2–3, ≥4), age at first birth (nulliparous, <26, ≥26 years, missing), menopause status (premenopause, age at menopause <51, ≥51 years) and use of exogenous female hormones (never, ever).
Table 5 Breast cancer risk according to quintile (Q) of dietary pattern score with stratification by menopausal statusFootnote * (Hazard ratios (HR) and 95 % confidence intervals)

* Age 51 years was used as a proxy cut-off point for menopausal age, the time at which approximately 50 % of the women had become postmenopausal, as no study had direct information on menopausal status after the start of follow-up. P interaction value between menopausal status and each dietary pattern category were 0·4703 (prudent), 0·3542 (westernised), and 0·6152 (traditional), respectively, in the age- and area-adjusted model; and 0·5058 (prudent), 0·3492 (westernised), and 0·5146 (traditional), respectively, in the multivariable adjusted model.
† Based on Cox proportional hazards model, with ordinal numbers 0–4 assigned to the quintile categories of each dietary pattern.
‡ Multivariable adjusted: multivariable Cox proportional hazards model was adjusted for age (<50, 50–54·9, 55–59·9, 60–64·9, 65–69·9, ≥70 years), public health centre area (ten), log-transformed energy intake (continuous), BMI (<22·5, 22·5–24·9, 25–27·4, ≥27·5 kg/m2), smoking status (never, past, current), leisure-time physical activity (<1 d/month, 1–3 d/month, ≥1 d/week), total physical activity (<30, ≥30 metabolic equivalent task-h/d, missing), age at menarche (≤13, 14, 15, ≥16 years, missing), parity (nulliparous, 1, 2–3, ≥4), age at first birth (nulliparous, <26, ≥26 years, missing), menopause status (premenopause, age at menopause <51, ≥51 years), use of exogenous female hormones (never, ever).
Table 6 Breast cancer risk according to quintiles (Q) of dietary pattern score by hormone receptor status (Hazard ratios (HR) and 95 % confidence intervals)

ER+, oestrogen receptor positive; PR+, progesterone receptor positive; Ref., referent values; ER–, oestrogen receptor negative; PR–, progesterone receptor negative.
* Based on Cox proportional hazards model, with ordinal numbers 0–4 assigned to the quintile categories of each dietary pattern.
† Multivariable adjusted: multivariable Cox proportional hazards model was adjusted for age (<50, 50–54·9, 55–59·9, 60–64·9, 65–69·9, ≥70 years), public health centre area (ten), log-transformed energy intake (continuous), BMI (<22·5, 22·5–24·9, 25–27·4, ≥27·5 kg/m2), smoking status (never, past, current), leisure-time physical activity (<1 d/month, 1–3 d/month, ≥1 d/week), total physical activity (<30, ≥30 metabolic equivalent task-h/d, missing), age at menarche (≤13, 14, 15, ≥16 years, missing), parity (nulliparous, 1, 2–3, ≥4), age at first birth (nulliparous, <26, ≥26 years, missing), menopause status (premenopause, age at menopause <51, ≥51 years) and use of exogenous female hormones (never, ever).
In analyses stratified by menopausal status, although the positive association between the westernised dietary pattern and breast cancer risk was not significant in the full model adjusted for covariates, postmenopausal women in the highest quintile of the westernised dietary pattern had a 29 % increase in risk of breast cancer (95 % CI 0·99, 1·76; P trend=0·04) (Table 5). In subanalyses that considered hormone receptor status (ER, PR), the association between the westernised dietary pattern and breast cancer risk was statistically significant only for ER+/PR+ tumours (for Q5 v. Q1, multivariable HR 2·49; 95 % CI 1·40, 4·43; P trend<0·01) (Table 6).
No clear association was found between breast cancer risk and the prudent and traditional Japanese dietary patterns in Japanese women.
Discussion
In this study, we identified three dietary patterns in a large sample of Japanese women, which we labelled prudent, westernised and traditional dietary patterns. We found that the westernised dietary pattern was associated with an increased risk of breast cancer. This increased risk was more pronounced in women with extreme intake of a western diet (Q5_5th category of the westernised dietary pattern), postmenopausal women and for ER+/PR+ tumours.
Results from previous studies on the association between dietary pattern and breast cancer risk have been inconsistent. Most previous prospective analyses were conducted in Western populations( Reference Velie, Schairer and Flood 8 , Reference Adebamowo, Hu and Cho 9 , Reference Sieri, Krogh and Pala 11 , Reference Sant, Allemani and Sieri 12 , Reference Fung, Hu and Holmes 17 , Reference Baglietto, Krishnan and Severi 26 – Reference Terry, Suzuki and Hu 29 ), and little prospective data from Asian populations with a western dietary pattern have been reported. In agreement with our findings, a previous prospective cohort study of the association between dietary pattern and breast cancer risk in France found that a western dietary pattern was positively associated with breast cancer risk (HR=1·20), especially in women with ER+/PR+ tumours( Reference Cottet, Touvier and Fournier 30 ). Only one Asian prospective cohort study reported a trend for lower breast cancer risk in those with higher intake of a vegetable–fruit–soya (prudent) dietary pattern, with particularly stronger effects among postmenopausal women( Reference Butler, Wu and Wang 31 ).
In the present study, the westernised dietary pattern was positively associated with breast cancer risk. Identifying the specific components (nutrients or foods) of the westernised dietary pattern that contribute to this positive association with breast cancer risk is difficult. The westernised dietary pattern in our study was characterised by high intakes of meats, processed meats, dairy products, several beverages and sauces, and alcohol, but a low intake of vegetables and fruits. A refined grain–meat–pickles pattern in Chinese women( Reference Zhang, Ho and Fu 32 ) and a fatty dietary pattern in Japanese women( Reference Hirose, Matsuo and Iwata 10 ) were positively associated with breast cancer risk. A recent meta-analysis of studies concluded that the risk of breast cancer for the highest v. lowest categories increased by 10 % for red meat and 8 % for processed meat( Reference Guo, Wei and Zhan 33 ). It has been hypothesised that diets with a high intake of meat and processed meat promote carcinogenesis via certain carcinogenic compounds such as heterocyclic amines (HCA) and polycyclic aromatic hydrocarbons (PAH), which are by-products of cooking meat at high temperatures( Reference Knize, Salmon and Pais 34 , Reference Kazerouni, Sinha and Hsu 35 ). Evidence for positive associations between HCA and PAH intake and overall breast cancer risk has been identified in human studies( Reference Bonner, Han and Nie 36 , Reference Zheng and Lee 37 ).
In addition, a World Cancer Research Fund/American Institute for Cancer Research report( 38 ) states that alcohol consumption is a risk factor for breast cancer, and most epidemiological studies have consistently identified an association between breast cancer risk and alcohol intake( Reference Smith-Warner, Spiegelman and Adami 39 , Reference Tjonneland, Christensen and Olsen 40 ). Several biological mechanisms for this association have been proposed, including an increase in circulating hormone levels, direct carcinogenic effects of alcohol metabolites such as acetaldehyde and an antagonistic effect on folate absorption and metabolism( Reference Zhang, Willett and Selhub 41 , Reference Lew, Freedman and Leitzmann 42 ). Furthermore, dietary behaviours such as frequent consumption of sweet soda, coffee and tea with added sugar and sauces with high fat and sugar content increase blood glucose levels. Hyperinsulinemia may be a risk factor for breast cancer as insulin is mitogenic, and thereby encourages cellular proliferation and promotes tumour growth; excess insulin is also indirectly related to increased levels of free oestrogen via inhibition of the production of sex-hormone-binding globulin( Reference Bradshaw, Sagiv and Kabat 43 ).
Considered broadly, these various findings may suggest that one reason for the positive association between the westernised dietary pattern and risk of breast cancer is the complex or synergistic effects achieved by combining food/food groups on breast cancer. In addition, the combined effects of dietary pattern on breast cancer likely reflect overall dietary behaviours, which may in turn explain a mixture of social, cultural, environmental, health, economic and lifestyle factors( Reference Reedy, Wirfalt and Flood 44 ). In the present study, we could not obtain consistent findings for any associations of westernised dietary pattern with risk of breast cancer among premenopausal women. The possible differential effect by menopausal status could be due to the influence of genetic factors and early-life events, which are stronger for premenopausal breast cancer( Reference Lagiou, Hsieh and Trichopoulos 45 , Reference Loman, Johannsson and Kristoffersson 46 ). For example, a prospective study performed on breast cancer in Italy reported that a higher ratio of 2-hydroxyestrone (lower oestrogenic activity):16α-hydroxyestrone (biologically strong oestrogens) was associated with reduced risk of breast cancer among premenopausal women, but not in postmenopausal women( Reference Muti, Bradlow and Micheli 47 ). Furthermore, age-related changes in local tissue metabolism of sex steroids such as oestrogen, testosterone and progesterone( Reference Hankinson and Eliassen 48 ) may be partially explained by some differences between dietary factor and breast cancer risk in premenopausal and postmenopausal women. Another possible reason is that the lack of statistical significance in premenopausal women might be due to chance, given the relatively small number of cases of breast cancer in our premenopausal subjects. In our study, the westernised dietary pattern had a strong positive effect on ER+/PR+ tumours. Although a few studies have assessed dietary patterns and nutrients with regard to ER/PR subtype, two studies reported a significant inverse association for the prudent dietary pattern and ER- breast cancer in black women( Reference Agurs-Collins, Rosenberg and Makambi 27 ) and in postmenopausal women in the Nurses’ Health Study( Reference Fung, Hu and Holmes 17 ). Regarding components of the westernised diet, alcohol intake( Reference Suzuki, Ye and Rylander-Rudqvist 49 ) and excess dietary fat( Reference Kushi, Potter and Bostick 50 , Reference Sieri, Chiodini and Agnoli 51 ) were more strongly associated with ER+ than ER– tumours, although other studies found no evidence of an association with dietary fat by ER/PR status( Reference Park, Kolonel and Henderson 52 , Reference Kim, Willett and Colditz 53 ). Thus, future aetiological studies of breast cancer should stratify analyses by tumour receptor type.
The major strengths of this study include its large sample size and population-based prospective design, in which information was collected before the subsequent diagnosis of breast cancer, thereby avoiding the exposure recall bias inherent to case–control studies. Our subjects were recruited from the general population, the sample was large, the response rate to the questionnaire was more than 80 % and loss to follow-up was a negligible 0·4 %( Reference Tsugane and Sawada 19 ). In addition, the cancer registry of the study population was of sufficient quality to reduce the possibility of misclassification of outcomes. To examine the association between breast cancer risk and dietary factors, we used the dietary pattern method to identify the overall dietary habits of our subjects. This approach is particularly useful when planning dietary preventive strategies or carrying our nutritional education, because it considers the overall diet, and therefore encompasses complex relations, potential interactions and synergetic effects among nutrients, food and other food components.
Several limitations to the present study warrant mention. First, principal component analyses require several subjective decisions in determining the number of factors to be retained, the method of rotation of the initial factors and the labelling of dietary patterns( Reference Hu 18 , Reference Martinez, Marshall and Sechrest 54 ). Although the dietary patterns in this study cannot be compared directly with those of other studies because of differences in the process, they are similar to those reported previously among Japanese( Reference Kashino, Nanri and Kurotani 55 , Reference Kurotani, Kochi and Nanri 56 ). Moreover, we confirmed that the validity and reproducibility of the three dietary patterns derived from subsamples were reasonable( Reference Nanri, Shimazu and Ishihara 24 ). Second, we assessed the usual dietary intake only at one time point, and long-term dietary changes may not be accurately reflected during the follow-up period. Third, although we examined and adjusted for several potential confounders in the statistical model, the possible effects of confounding by unmeasured variables and residual confounding cannot be totally discarded.
In summary, we found that a westernised dietary pattern with high intake of meat, processed meat, bread, dairy products, coffee, soft drinks, tea, sauces and alcohol was associated with an increase in the risk of breast cancer in a large sample of Japanese women. In particular, the increased risk associated with this dietary pattern was more pronounced among those women with extremely high intake (top of 4 %) of a western diet, those who were postmenopausal and those with ER+/PR+ tumours. This increased risk with the western dietary pattern in this cohort study may be due to the intake of specific combinations of food or food components, the diet pattern as a whole or other unconsidered characteristics of individuals who consume this diet. Therefore, these findings may indicate a potential avenue for public health intervention.
Acknowledgements
This study was supported by the National Cancer Center Research and Development Fund (23-A-31(toku) and 26-A-2) (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010). S. Shin was supported by the basic science research programme through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2014R1A6A3A03056928). M. Inoue is the beneficiary of a financial contribution from the AXA Research Fund as chair holder of the AXA Department of Health and Human Security, Graduate School of Medicine, The University of Tokyo. The AXA Research Fund had no role in the design, data collection, analysis, interpretation or drafting of the manuscript, or in the decision to submit the manuscript for publication.
The author responsibilities were as follows – S. Shin analysed the data and drafted the manuscript; M. Inoue, S. T., E. S., N. S., T. S., T. Y., M. Iwasaki, S. Sasazuki, J. I., R. T. and A. N. reviewed and edited the manuscript and contributed to the discussion; M. Inoue and S. T. conducted, designed and supervised the study; and all the authors read and approved the final version of the manuscript.
None of the authors reported any conflicts of interest related to the study.
Appendix
The members of the Japan Public Health Center-based Prospective Study (principal investigator: S. Tsugane) Group are as follows: S. Tsugane, N. Sawada, M. Iwasaki, S. Sasazuki, T. Yamaji, T. Shimazu and T. Hanaoka, National Cancer Center, Tokyo; J. Ogata, S. Baba, T. Manami, A. Okayama, and Y. Kokubo, National Cerebral and Cardiovascular Center, Osaka; K. Miyakawa, F. Saito, A. Koizumi, Y. Sano, I. Hashimoto, T. Ikuta, Y. Tanaba, H. Sato, Y. Roppongi, and T. Takashima, Iwate Prefectural Ninohe Public Health Center, Iwate; Y. Miyajima, N. Suzuki, S. Nagasawa, Y. Furusugi, N. Nagai, Y. Ito, S. Komatsu and T. Minamizono, Akita Prefectural Yokote Public Health Center, Akita; H. Sanada, Y. Hatayama, F. Kobayashi, H. Uchino, Y. Shirai, T. Kondo, R. Sasaki, Y. Watanabe, Y. Miyagawa, Y. Kobayashi, M. Machida, K. Kobayashi and M. Tsukada, Nagano Prefectural Saku Public Health Center, Nagano; Y. Kishimoto, E. Takara, T. Fukuyama, M. Kinjo, M. Irei, and H. Sakiyama, Okinawa Prefectural Chubu Public Health Center, Okinawa; K. Imoto, H. Yazawa, T. Seo, A. Seiko, F. Ito, F. Shoji and R. Saito, Katsushika Public Health Center, Tokyo; A. Murata, K. Minato, K. Motegi, T. Fujieda and S. Yamato, Ibaraki Prefectural Mito Public Health Center, Ibaraki; K. Matsui, T. Abe, M. Katagiri, M. Suzuki, K. and Matsui, Niigata Prefectural Kashiwazaki and Nagaoka Public Health Center, Niigata; M. Doi, A. Terao, Y. Ishikawa, and T. Tagami, Kochi Prefectural Chuo-higashi Public Health Center, Kochi; H. Sueta, H. Doi, M. Urata, N. Okamoto, and F. Ide and H. Goto, Nagasaki Prefectural Kamigoto Public Health Center, Nagasaki; H. Sakiyama, N. Onga, H. Takaesu, M. Uehara, T. Nakasone and M. Yamakawa, Okinawa Prefectural Miyako Public Health Center, Okinawa; F. Horii, I. Asano, H. Yamaguchi, K. Aoki, S. Maruyama, M. Ichii, and M. Takano, Osaka Prefectural Suita Public Health Center, Osaka; Y. Tsubono, Tohoku University, Miyagi; K. Suzuki, Research Institute for Brain and Blood Vessels Akita, Akita; Y. Honda, K. Yamagishi, S. Sakurai and N. Tsuchiya, University of Tsukuba, Ibaraki; M. Kabuto, National Institute for Environmental Studies, Ibaraki; M. Yamaguchi, Y. Matsumura, S. Sasaki, and S. Watanabe, National Institute of Health and Nutrition, Tokyo; M. Akabane, Tokyo University of Agriculture, Tokyo; T. Kadowaki and M. Inoue, The University of Tokyo, Tokyo; M. Noda and T. Mizoue, National Center for Global Health and Medicine, Tokyo; Y. Kawaguchi, Tokyo Medical and Dental University, Tokyo; Y. Takashima and Y. Yoshida, Kyorin University, Tokyo; K. Nakamura and R. Takachi, Niigata University, Niigata; J. Ishihara, Sagami Women’s University, Kanagawa; S. Matsushima and S. Natsukawa, Saku General Hospital, Nagano; H. Shimizu, Sakihae Institute, Gifu; H. Sugimura, Hamamatsu University School of Medicine, Shizuoka; S. Tominaga, Aichi Cancer Center, Aichi; N. Hamajima, Nagoya University, Aichi; H. Iso and T. Sobue, Osaka University, Osaka; M. Iida, W. Ajiki, and A. Ioka, Osaka Medical Center for Cancer and Cardiovascular Disease, Osaka; S. Sato, Chiba Prefectural Institute of Public Health, Chiba; E. Maruyama, Kobe University, Hyogo; M. Konishi, K. Okada, and I. Saito, Ehime University, Ehime; N. Yasuda, Kochi University, Kochi; S. Kono, Kyushu University, Fukuoka; S. Akiba, Kagoshima University, Kagoshima.











