Abstract
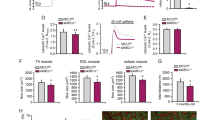
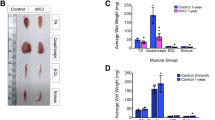
We have blocked creatine kinase (CK) mediated phosphocreatine (PCr) ⇄ ATP transphosphorylation in mitochondria and cytosol of skeletal muscle by knocking out the genes for the mitochondrial (ScCKmit) and the cytosolic (M-CK) CK isoforms in mice. Animals which carry single or double mutations, if kept and tested under standard laboratory conditions, have surprisingly mild changes in muscle physiology. Strenuous ex vivo conditions were necessary to reveal that MM-CK absence in single and double mutants leads to a partial loss of tetanic force output. Single ScCKmit deficiency has no noticeable effects but in combination the mutations cause slowing of the relaxation rate. Importantly, our studies revealed that there is metabolic and cytoarchitectural adaptation to CK defects in energy metabolism. The effects involve mutation type-dependent alterations in the levels of AMP, IMP, glycogen and phosphomonoesters, changes in activity of metabolic enzymes like AMP-deaminase, alterations in mitochondrial volume and contractile protein (MHC isoform) profiles, and a hyperproliferation of the terminal cysternae of the SR (in tubular aggregates). This suggests that there is a compensatory resiliency of loss-of-function and redirection of flux distributions in the metabolic network for cellular energy in our mutants.
Similar content being viewed by others
References
Bessmann SP, Carpenter LC: The creatine-creatine phosphate energy shuttle. Annu Rev Biochem 54: 831–862, 1985
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM: Intracellular compartmentalization, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The phosphocreatine circuit for cellular energy homeostasis. Biochem J 281: 21–40, 1992
Miller DS, Horowitz SB: Intracellular compartmentalization of adenosine triphosphate. J Biol Chem 261: 13911–13915, 1986
Saks VA, Khuchua ZA, Vasilyeva EV, Belikova OY, Kuznetsov AV: Metabolic compartmentalization and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration–a synthesis. Mol Cell Biochem 133/134: 155–192, 1994
Han J-W, Thieleczek R, Varsányi M, Heilmeyer LMG: Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry 31: 377–384, 1992
Rossi AM, Eppenberger HM, Volpe P, Cotrufo R, Wallimann T: Muscletype MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem 265: 5258–5266, 1990
Hardin C, Raeymakers L, Paul RJ: Comparison of endogenous and exogenous sources of ATP in fuelling Ca uptake in smooth muscle plasma membrane vesicles. J Gen Physiol 99: 21–40, 1992
Korge P, Campbell KB: Local ATP regeneration is important for sarcoplasmic reticulum Ca2+ pump function. Am J Physiol 267: C357–C366, 1994
Minajeva A, Ventura-Clapier R, Veksler V: Ca2+ uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly depends on bound creatine kinase. Pfügers Arch Eur J Physiol 432: 904–912, 1996
Erecinska M, Wilson D: Regulation of cellular energy metabolism. J Membr Biol 70: 1–14, 1982
Balaban RS: Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol 258: C377–C389, 1990
Sistermans EA: Murine creatine kinase B: Cell-Type Specific Expression and Biochemical Role. Ph.D. Thesis, University Nijmegen, 1996
Steeghs K: Consequences of Creatine Kinase Deficiencies in Mice. Ph.D. Thesis, University of Nijmegen, 1995
Brdiczka D, Wallimann T: The importance of the outer mitochondrial compartment in regulation of energy metabolism. Mol Cell Biochem 133/134: 69–83, 1994
Fritz-Wolf K, Schnyder T, Wallimann T, Kabsch W: Structure of mitochondrial creatine kinase. Nature 381: 341–345, 1996
Jaenisch R: Transgenic animals. Science 240: 1468–1474, 1988
Thomas KR, Capecchi MR: Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51: 503–512, 1987
Brandon EP, Idzerda RL, McKnight GS: Targeting the mouse genome: A compendium of knockouts. Curr Biol 5: 625, 758 and 873, 1995
Capecchi MR: The new mouse transgenetics: Altering the genome by gene targeting. Trends Genet 5: 70–76, 1989
Evans MJ: Potential for genetic manipulation of mammals. Mol Biol Med 6: 557–565, 1989
Joyner AL: Gene targeting and gene trap screens using embryonic stem cells: New approaches to mammalian development. Bioessays 13: 649–656, 1991
Rossant J: Gene disruption in mammals. Curr Opin Genet Dev 1: 236–240, 1991
Smithies O: Animal models of human genetic diseases. Trends Genet 9: 112–117, 1993
Satoh S, Tanaka A, Hatano E, Inomoto T, Iwata S, Kitai T, Shinohara H, Tsunekawa S, Chance B, Yamaoka Y: Energy metabolism and regeneration in transgenic mouse liver expressing creatine kinase after major hepatectomy. Gastroenterology 110: 1166–1174, 1996
Koretsky AP: Insights into cellular energy metabolism from transgenic mice. Physiol Rev 75: 667–688, 1995
Brosnan MJ, Chen L, Van Dyke TA, Koretsky AP: Free ADP levels in transgenic mouse liver expressing creatine kinase: Effects of enzyme activity, phosphagen type, and substrate concentration. J Biol Chem 265: 20849–20855, 1990
Brosnan MJ, Raman SP, Chen L, Koretsky AP: Altering creatine kinase isoenzymes in transgenic mouse muscle by overexpression of the B subunit. Am J Physiol 264: C151–C160, 1993
Van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, ter Laak H, Wieringa B: Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74: 621–631, 1993
Van Deursen J, Ruitenbeek W, Heerschap A, Jap P, ter Laak H, Wieringa B: Creatine kinase in skeletal muscle energy metabolism: a study of mouse mutants with graded reduction in M-CK expression. Proc Natl Acad Sci USA 91: 9091–9095, 1994
Steeghs K, Peters W, Croes H, Bruckwilder M, van Alewijck D, Wieringa B: Mouse ubiquitous mitochondrial creatine kinase: Gene organization and consequences from inactivation in mouse embryonic stem cells. ONA Cell Biol 14: 539–553, 1995
Steeghs K, Heerschap A, de Haan A, Ruitenbeek W, Oerlemans F, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Wieringa B: Use of gene targeting for compromising energy homeostasis in neuromuscular tissues: The role for sarcomeric mitochondrial creatine kinase. J Neurosci Meth 71: 29–41, 1997
Levitsky DO, Levchenko TS, Saks VA, Sharov VG, Smirnov VN: The role of creatine phosphokinase in supplying energy for the calcium pump system of heart sarcoplasmic reticulum. Membr Biochem 2: 81–96, 1978
Saks VA, Lipina NV, Sharov VG, Smirnov VA, Chazov EI, Grosse R: The localization of MM-isoenzyme of creatine kinase on the surface membrane of myocardial cells and its functional coupling to ouabain-inhibited Na+/K+ ATPase. Biochim Biophys Acta 465: 550–558, 1977
Grosse R, Spitzer E, Kupriyanov VV, Saks VA, Repke KRH: Coordinate interplay between (Na+/K+)-ATPase and CK optimizes (Na+/K+)-antiport across the membrane of vesicles formed from the plasma membrane of cardiac muscle cells. Biochim Biophys Acta 603: 142–156, 1980
Wegmann G, Zanolla E, Eppenberger HM, Wallimann T: In situ compartmentalization of creatine kinase in intact sarcomeric muscle: The acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil 13: 420–435, 1992
Turner DC, Wallimann T, Eppenberger HM: A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci USA 70: 702–705, 1973
Ventura-Clapier R, Saks VA, Vassort G, Lauer C, Elizarova GV: Reversible MM-creatine kinase binding to cardiac muscle. Am J Physiol 253: C444–C455, 1987
Ventura-Clapier R, Veksler V, Hoerter JA: Myofibrillar creatine kinase and cardiac contraction. Mol Cell Biochem 133/134: 125–144, 1994
Sata M, Ugiura S, Yamashita H, Momomura S-i, Serizawa T: Coupling between myosin ATPase cycle and creatine kinase cycle facilitates cardiac actomysin sliding in vitro. Circulation 93: 310–317, 1996
Wyss M, Smeitink J, Wevers R, Wallimann T: Mitochondrial creatine kinase: A key enzyme of aerobic energy metabolism. Biochim Biophys Acta 1102: 119–166, 1992
Zeleznikar RJ, Dzeja PP, Goldberg ND: Adenylate kinase-catalyzed phosphoryl transfer couples ATP utilization with its generation by glycolysis in intact muscle. J Biol Chem 270: 7311–7319, 1995
Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B: Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell 89: 93–103, 1997
Kramer K, van Acker SABE, Voss H-P, Grimbergen JA, van der Vijgh WJF, Bast A: Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Meth 30: 209–215, 1993
Wan R, Diamant M, de Jong W, De Wied D: Changes in heart rate and body temperature during passive avoidance behaviour in rats. Physiol Behavior 47: 493–499, 1990
Gerlai R: Gene-targeting studies of mammalian behavior: Is it the mutation or the background genotype? TINS 19: 177–190, 1996
Dubowitz V: Muscle Biopsy: A Practical Approach. Lavenham Press, Lavenham, England, 1985
Biermans W, Bakker A, Jacob W: Contact sites between inner and outer mitochondrial membrane: A dynamic microcompartment for creatine kinase. Biochim Biophys Acta 1018: 225–228, 1990
Bakker A, Bernaert I, De Bie M, Ravingerova T, Ziegelhöffer A, Van Belle H, Jacob W: The effect of calcium on the mitochondrial contact sites: A study on isolated rat hearts. Biochim Biophys Acta 1224: 583–588, 1994
Baddeley AJ, Gundersen HJG, Cruz-Orive LM: Estimation of surface area from vertical sections. J Microscopy 142: 259–276, 1986
Salviati G, Pierobon-Bormioli S, Betto R, Damiani E, Angelini C, Ringel SP, Salvatori S, Margreth A: Tubular aggregates: Sarcoplasmic reticulum origin, calcium storage ability, and functional implications. Muscle and Nerve 8: 299–306, 1985
Hoffman EP, Lehmann-Horn F, Rudel R: Overexcited or inactive: Ion channels in muscle disease. Cell 80: 681–686, 1995
Meijer AEFH: Histochemical features of tubular aggregates in diseased human skeletal muscle fibers. J Neurol Sciences 86: 73–82, 1988
Rosenberg NL, Neville HE, Ringel SP: Tubular aggregates, their association with neuromuscular diseases, including the syndrome of myalgias/cramps. Arch Neurol 42: 973–976, 1985
Craig ID, Allen IV: Tubular aggregates in murine dystrophy heterozygotes. Muscle Nerve 3: 134–140, 1980
Kuncl RW, Pestronk A, Lane J, Alexander E: The MRL +/+ mouse: A new model of tubular aggregates which are gender-and age related. Acta Neuropathol 78: 615–620, 1989
Stephanopoulos G, Vallino JJ: Network rigidity and metabolic engineering in metabolite overproduction. Science 252: 1675–1681, 1991
Tullson PC, Arabadjis PG, Rundell KW, Terjung RL: IMP reamination in rat skeletal muscle fiber types. Am J Physiol 270: C1069–C1074 (Cell Physiol. 39), 1996
Sahlin K, Gorski J, Edstrom L: Influence of ATP turnover and metabolite changes on IMP formation and glycolysis in rat skeletal muscle. Am J Physiol 259: C409–C412 (3 Pt 1), 1990
Tullson PC, Wieringa B, Terjung RL: Skeletal muscle creatine kinase deficiency decreases AMP deaminase activity and apparent molecular weight. Med Sci Sports Exer 28: S76, 1996
Tullson PC, Rundell KW, Sabina RL, Terjung RL: Treatment with the creatine analog β-guanidinoproprionic acid alters skeletal muscle AMP deaminase activity in vitro and in vivo. Am J Physiol 270: C76–C85 (Cell Physiol), 1996
Sweeney HL, Kushmerick MJ, Mabuchi K, Sreter FA, Gergely J: Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem 263: 9034–9039, 1988
Pette D, Vrbova G: Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol 120: 115–202, 1992
Van Deursen J, Wieringa B: Targeting of the creatine kinase M gene in embryonic stem cells using isogenic and nonisogenic vectors. Nucl Acids Res 20: 3815–3820, 1992
Heerschap A, Bergman AH, Vaals JJ, Wirtz P, Loermans HMT, Veerkamp JH: Alterations in relative phosphocreatine concentrations in preclinical mouse muscular dystrophy revealed by in vivo NMR. NMR Biomed 1: 27–31, 1988
Heerschap A, van Vaals JJ, Bergman AH, Den Boer JH, van Gerwen PHJ, Gravenmade EJ: In vivo 31P-NMR studies of rat salivary glands. Magn Reson Med 8: 129, 1988
De Haan A, Jones DA, Sargeant AJ: Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius. Pflügers Arch 413: 422–428, 1989
Edwards RHT, Hill DK, Jones DA: Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol 251: 287–301, 1975
Hämäläinen N, Pette D. The histochemical profiles of fast fiber types JIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41: 733–743, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steeghs, K., Oerlemans, F., de Haan, A. et al. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem 184, 183–194 (1998). https://doi.org/10.1023/A:1006811717709
Issue Date:
DOI: https://doi.org/10.1023/A:1006811717709