Abstract
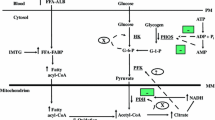
Long chain free fatty acids (FFA) are the preferred metabolic substrates of myocardium under aerobic conditions. However, under ischemic conditions long chain FFA have been shown to be harmful both clinically and experimentally. Serum levels of free fatty acids frequently are elevated in patients with myocardial ischemia. The proposed mechanisms of the detrimental effects of free fatty acids include: (1) accumulation of toxic intermediates of fatty acid metabolism, such as long chain acyl-CoA thioesters and long chain acylcarnitines, (2) inhibition of glucose utilization, particularly glycolysis, during ischemia and/or reperfusion, and (3) uncoupling of oxidative metabolism from electron transfer. The relative importance of these mechanisms remains controversial. The primary site of FFA-induced injury appears to be the sarcolemmal and intracellular membranes and their associated enzymes. Inhibitors of free fatty acid metabolism have been shown experimentally to decrease the size of myocardial infarction and lessen postischemic cardiac dysfunction in animal models of regional and global ischemia. The mechanism by which FFA inhibitors improve cardiac function in the postischemic heart is controversial. Whether the effects are dependent on decreased levels of long chain intermediates and/or enhancement of glucose utilization is under investigation. Manipulation of myocardial fatty acid metabolism may prove beneficial in the treatment of myocardial ischemia, particularly during situations of controlled ischemia and reperfusion, such as percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. (Mol Cell Biochem 166: 85-94, 1997)
Similar content being viewed by others
References
Oliver MF, Kurien VA, Greenwood TW: Relation between serum free-fatty acids and arrythmias and death after acute myocardial infarction. Lancet 1: 710–715, 1968
Gupta DK, Jewitt DE, Young R, Hartog M, Opie LH: Increased plasma-free-fattyacid concentrations and their significance in patients with acute myocardial infarction. Lancet 2: 1209–1213, 1969
Storstein L, Nitter-Hauge S, Fjeld N: Effects of cardiopulmonary bypass with heparin administration on digoxin pharmacokinetics, serum electrolytes, free fatty acids and renal function. J Cardiovasc Pharm 1: 191–204, 1979
Opie LH: Metabolism of free fatty acids, glucose and catecholamines in acure myocardial infarction. Am J Cardiol 36: 938–953, 1975
Svennson S, Svedjeholm R, Ekroth R, Milocco I, Nilsson F, Sabel KG, WilliamOlsen G: Trauma metabolism and the heart. J Thorac Cardiovasc Surg 99: 1063–1073, 1990
Lopaschuk GD, Collins-Nakai P, Olley PM, Montague TJ, McNeil G, Gayle M, Penkaske P, Finegan BA: Plasma free fatty acid levels in infants and adults after myocardial ischemia. Am Heart J 128: 61–67, 1994
Reves JG, Karp RB, Buttner EE, Tosone S, Smith LR, Samuelson PN, Kreusch GR, Oparil S: Neuronal and adrenomedullary catecholamine release in response to cardiopulmonary bypass in man. Circulation 66: 49–55, 1982
Molaparast-Saless F, Liedtke AJ, Nellis SH: Effects of fatty acid blocking agents oxfenicine and 4-bromocrotonic acid in aerobic and ischemic myocardium. J Mol Cell Cardiol 19: 509–520, 1987
Neely JR, Morgan HE: Relationship between carbohydrate and lipid metabolism and the energy balance of the heart. Ann Rev Physiol 36: 413–459, 1974
Van der Vusse GJ, Glatz JF, Stam HCG, Reneman R: Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72: 881–940, 1992
Liedtke AJ: Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis 23: 321–336, 1981
Knudsen J, Mandrup S, Rasmusson JT, Andreason PH, Poulson F, Kristiansen K: The function of acyl-CoA-binding protein (ACBP)/diazepam binding inhibitor (DBI). Mol Cell Biochem 123: 129–138, 1993
Waku K: Origins and fates of fatty acyl-CoA esters. Biochim Biophys Acta 1124: 101–111, 1992
Fournier NC, Rahim M: Control of energy production in the heart: a new function for fatty acid binding protein. Biochem 24: 2387–2396, 1985
Lopashuck GD, Belke DD, Gamble J, Itoi T, Schonekess BO: Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213: 263–276, 1994
Saddik M, Lopaschuk GD: Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266: 8162–8170, 1991
Murthy MSR, Pande SV: Malonyl-CoA binding site and the overt carnitine palmitoyltraansferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci 84: 378–382, 1987
Schulz H: Regulation of fatty acid oxidation in heart. J Nutr 124: 165–171, 1994
Olowe Y, Schulz H: Regulation of thiolases from pig heart. Control of fatty acid oxidation in heart. Eur J Biochem 109: 425–429, 1980
Forster ME, Staib W: β-oxidation as a channeled reaction linked to the citric acid cycle: evidence from measurements of mitochondrial pyruvate oxidation during fatty acid degradation. Int J Biochem 24: 1111–1116, 1992
Sumeji B, Porpaczy Z, Alkonyi I: Kinetic advantage of the interaction between the fatty acid β-oxidation enzymes and the complexes of the respiratory chain. Biochim Biophys Acta 1081: 121–128, 1991
Watmaugh NJ, Turnbull DM, Sheratt HS, Bartlett K: Measurement of the acylCoA intermediates of β-oxidation by high performance liquid chromatography with on-line radiochemical and photodiode-array detection. Application to the study of [U-14C] hexadecanoate oxidation by intact rat liver mitochondria. Biochem J 262: 261–269, 1989
Nada MA, Rhead WJ, Sprecher H, Schulz H, Roe CR: Evidence for intermediate channeling in mitochondrial β-oxidation. J Biol Chem 270: 530–535, 1995
Opie LH: Fuels: carbohydrates and lipids. In: The heart: physiology and metabolism. 2nd ed. Raven Press, New York, 1991, pp 208–246
Pearce FJ, Forster J, DeLeeuw G, Williamson JR, Tutwiler GF: Inhibition of fatty acid oxidation in normal and hypoxic perfused rat hearts by 2-tetradecylglycidic acid. J Mol Cell Cardiol 11: 893–915, 1979
Hutter JP, Schweickhardt C, Piper HM, Spieckerman PG: Inhibition of fatty acid oxidation and decrease of oxygen consumption of working rat heart by 4bromocrotonic acid. J Mol Cell Cardiol 16: 105–108, 1984
Randle PJ, Garland PB, Hales CN, Newsholme EA: The glucose-fatty acid cycle: its role in insulin insensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963
Saddik M, Gamble J, Witters LA, Lopaschuk GD: Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 268: 25836–25845, 1993
Lysiak W, Toth PP, Suelter CH, Bieber LL: Quantitation of the efflux of acylcarnitines from rat heart, brain, and liver mitochondria. J Biol Chem 261: 13698–13703, 1986
Cook GA: Differences in the sensitivity of carnitine palmitolytransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem 259: 12030–12033, 1984
Uziel G, Garavaglia B, DiDonato S: Carnitine stimulation of pyrovate dehydrogenase complex (PDHC) in isolated human skeletal muscle mitochondria. Muscle Nerve 11: 720–724, 1988
Broderick TL, Quinney HA, Lopaschuk GD: Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart J Biol Chem 267: 3758–3763, 1992
Ahdel-aleem S, Sayed-Ahmed M, Nada M, Hendrickson SC, St. Louis JD, Walthall HP, Lowe JE: Regulation of fatty acid oxidation by acetyl-CoA generated from glucose utilization in isolated myocytes. J Mol Cell Cardiol (in press)
Liedtke AJ: Alternations of carbohydrate and lipid metabolism in the acutely ischemic heart. Progress CV Dis 23: 321–336, 1981
Van Bilsen M, van der Vusse GJ, Willemsem PHM, Coumans WA, Roemen THM, Reneman RS: Lipid alterations in isolated, working rat hearts during ischemia and reperfusion: its relation to myocardial damage. Circ Res 64: 304–314, 1989
Lowe JE, Jennings RB, Reimer KA: Cardiac rigor mortis in dogs. J Mol Cell Cardiol 11: 1017–1031, 1979
Weiss J, Hiltbrand B: Functional compartmentalization of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest 75: 436–447, 1985
Owen P, Dennis S, Opie LH: Glucose flux rate regulates onset of ischemic contracture in globally underperfused rat hearts. Circ Res 66: 344–354, 1990
Meerson FZ, Kagan VE, Kozlov YP, Belkina LM, Arkhipenko YV: The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res Cardiol 77: 465–485, 1982
Mak IT, Kramer JH, Weslicki WB: Potentiation of free radical-induced lipid peroxidative injury to sarcolemmal membranes by lipid amphiphiles. J Biol Chem 261: 1153–1157, 1986
Liedtke AJ, Mahar CQ, Ytrehus K, Mjos OD: Estimates of free-radical production in rat and swine hearts: method and application of measuring malondialdohyde levels in fresh and frozen myocardium. Bas Res Cardiol 79: 513–518, 1984
Schwaiger M, Schelbert HR, Keen R, Vinten-Johansen J, Hansen H, Selin C, Barrio J, Huang SC, Phelps ME: Retention and clearance of C-11 palmitate in ischemic and reperfused canine myocardium. J Am Coll Cardiol 6: 311–320, 1985
Schwaiger M, Scheibert HR, Ellison D, Hansen H, Yeatman L, Vinten-Johansen J, Selin C, Barrio J, Phelps ME: Sustained regional abnormalities in cardiac metabolism after transient ischemia in the chronic dog model. J Am Coll Cardiol 6: 336–347, 1985
Liedtke AJ, DeMaison L, Eggleston AM, Cohen LM, Nellis SH: Changes in substrate merabolism and effects of excess fatty acids in reperfused myocardium. Circ Res 62: 535–542, 1988
Lopaschuk GD, Spafford MA, Davies NJ, Wall SR: Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res 66: 546–553, 1990
Gorge G, Chatelain P, Schaper J, Lerch R: Effect of increasing degrees of ischemic injury on myocardial oxidative metabolism early after reperfusion in isolated rat hearts. Circ Res 68: 1681–1692, 1991
Saddik M, Lopaschuk GD: Myocardial triglyceride turnover during reperfusion of isolated rat hearts subjected to a transient period of global ischemia. J Biol Chem 267: 3825–3831, 1992
Liedtke AJ, Nellis SH, Neely JR: Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ Res 43: 652–661, 1978
Katz AM, Mesineo FC: Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res 48: 1–16, 1981
Raz A, Livne A: Differential effects of lipids on the osmotic fragility of erythrocytes. Biochim Biophys Acta 311: 222–229, 1973
Adams RJ, Cohen DW, Gupte S, Johnson JD, Wallick ET, Wang T, Schwartz A: In vitro effects of palmitoylcarnitine on cardiac plasma membrane Na+,K+-ATPase and sarcoplasmic reticulum calcium ATPase and calcium transport. J Biol Chem 254: 12404–12410, 1979
Dhalla NS, Kolar F, Shah KR, Ferrari R: Effects of some L-carnitine derivatives on heart membrane ATPases. Cardiovasc Drugs Ther 5: 25–30, 1991
Osornio-Vargas AR, Berezesky JK, Trump BF: Progression of ion movements during acute myocardial infarction in the rat. An X-ray microanalysis study. Scan Electron Microse 2: 463–472. 1981
Adams RJ, Cohen DW, Gupte S, Johnson JD, Wallick ET, Wang T, Schwartz A: In vitro effects of palmitoylcarnitine on cardiac plasma membrane Na,K-ATPase, and sarcoplasmic reticulum Ca2+-ATPase and Ca2+ transport. J Biol Chem 264: 12404–12410, 1979
Abe M, Yamazaki N, Suzuki Y, Kobayashi A, Ohta A: Effects of palmitoylcarnitine on Na+/K+-ATPase and adenylate cyclase activity of canine myocardial sarcolemma. J Mol Cell Cardiol 16: 239–245, 1984
Shug AL, Shrago E, Bittar N, Folts JD, Koke JR: Acyl-CoA inhibition of adenine nucleotide translocase in ischemic myocardium. Am J Physiol 228: 689–692, 1975
Wolkowicz PE, Pownall HJ, McMillan-Wood JB: (1-Pyrenebutyryl)carnitine and 1-pyrenebutyryl coenzyme A: fluorescent probes for lipid metabolite studies in artificial and natural membranes. Biochemistry 21: 2990–2996, 1982
Wojtczak L: Effect of fatty acids and acyl-CoA on the permeability of mitochondrial membranes to monovalent cations. FEBS Lett 44: 25–30, 1974
McMillan-Wood J, Bush B, Pitts BJR, Schwartz A: Inhibition of bovine heart Na+-K+-ATPase by palmitoylcarnitine and palmitoyl-CoA. Biochim Biophys Res Commun 74: 677–684, 1977
Shrago E: Myocardial adenine nucleotide translocase. J Mol Cell Cardiol 8: 497–500, 1976
Messino FC, Rathier M, Favreau C, Wattras J, Takenaka H: Mechanisms of fatty acid effects on sarcoplasmic reticulum: III The effects of palmitic and oleic acids on sarcoplasmic reticulum function — a model for fatty acid membrane interactions. J Biol Chem 259: 1336–1343, 1984
Steigen TK, Aasum E, Myrmel T, Larsen TS: Effects of fatty acids on myocardial calcium control during hypothermic perfusion. J Thorac Cardiovasc Surg 107: 233–241, 1994
Watras J, Messineo FC, Herbette LG: Mechanisms of fatty acid effects o n sarcoplasmic reticulum: I. calcium-fatty acid interaction. J Biol Chem 259: 1319–1324, 1984
Feuvray D, Plouet J: Relationship between structure and fatty acid motabolism in mitochondria isolated from ischomic rat hearts. Circ Res 48: 740–747, 1981
Lamers JJM, De Jonge-Stinis JT, Verdouw PD, Hulsmann WC: On the possible role of long chain fatty acylcarnitine accumulation in producing functional and calcium permeability changes in membranes during myocardial ischemia. Cardiovasc Res 21: 313–322, 1987
Pitts BJR, Okhuysen CH: Effects of palmitoylcarnitine and LPC on cardiac sarcolemmal Na+-K+ ATPase. Am J Physiol 247: H840-H846, 1984
Weiss JN, Lamp ST: Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiomyocytes. Science 238: 67–69, 1987
Hardin C, Raeymakers L, Wuytack F, Casteels R, Paul RJ: An endogenous glycolytic cascade can preferentially fuel Ca2+-uptake in a plasma membrane vesicle fraction(PMV) of smooth muscle. Fed Proc 46: 1096, 1987, [Abstract]
Apstein CS, Gravino FN, Haudenschild CC: Determinants of the protective effect of glucose and insulin in ischemic myocardium: effects on contractile function, diastolic compliance, metabolism and ultrastructure during ischemia and reperfusion. Circ Res 52: 515–526, 1983
Marako PR, Libby P, Sobel BE, Bloor CM, Sybers HD, Shell WE, Correl JW, Braunwald E: Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circ 45: 1160–1175, 1972
Lazar HL, Zhang X, Rivers S, Bernard S, Shemin RJ: Limiting ischemic myocardial damage using glucose-insulin-potassium solutions. Ann Thorac Surg 60: 411–416, 1995
Neely JR, Grotyohann LW: Role of glycolytic products in damage to ischemic myocardium. Circ Res 55: 816–824, 1984
McVeigh JJ, Lopasohuk GD: Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol 259: H1079-H1085, 1990
Lopaschuk GD, Wall SR, Olley PM, Davies NJ: Etoxomir, a carnitine palmitoyltransferase inhibitor, protects hearts from fatty-acid induced ischemic injury independent of changes in long-chain acylcarnitine. Circ Res 63: 1036–1043, 1988
Jeremy RW, Ambrosio G, Pike MM, Jacobus WE, Becker LC: The functional recovery of post-ischemic myocardium requires glycolysis during early reperfusion. J Mol Cell Cardiol 25: 261–276, 1993
Gradinac S, Coleman GM, Taegtmeyer H, Sweeney MS, Frazier OH: Improved cardiac function with glucose-insulin-potassium after aortocoronary bypass grafting. Ann Thorac Surg 48: 484–489, 1989
Huang XQ, Liedtke AJ: Alterations in fatty acid oxidation in ischemic and reperfused myocardium. Mol Cell Biochem 88: 145–153, 1989
Wojtozak L, Schenfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta 1183: 41–57, 1993
Rottenberg H, Hashimoto K: Fatty acid uncoupling of oxidative phosphorylation in rat liver mitochondria. Biochem 25: 1747–1755, 1986
Bush LR, Shlafer M, Haack DW, Lucchesi BR: Time-dependent changes in canine cardiac mitochondrial funetion and ultrastrueture resulting from coronary occlusion and reperfusion. Bas Res Cardiol 75: 555–571, 1980
Wojtezak L, Bogucka K, Sarzala MG, Zaluska H: Effect of fatty acids on energy metabolism and the transport of adenine nucleotides in mitochendria and other cellular structures. In: L. Ernster and Z. Drahota (eds). Mitochondria, structure and function. Academic Press, New York, 1969, pp 79–92
Gutknecht J: Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J Membr Biol 106: 83–93, 1988
Schonfeld P, Schild L, Kunz W. Long-chain fatty acids act as protonophoric uncouplers of oxidative phosphorylation in rat liver mitochondria. Biochim Biophys Acta 977: 266–272, 1989
Andreyev AY, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Tsofina LM, Volkov NI, Vygodina TV: The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur J Biochem. 182: 585–592, 1989
Van Bilsen M, van der Vusse GJ, Willemsen PHM, Coumans WA, Roemen THM, Reneman RS: Lipid alterations in isolated, working rat hearts during ischemia and reperfusion: its relation to myocardial damage. Circ Res 64: 304–314, 1989
Vik mo H, Mjos OD, Neely JR, Mareko PR, Ribiero LGT: Limi tation of myocardial infarct size by metabolic interventions that reduce accumulation of fatty acid metabolites in ischemic myocardium. Am Heart J 111: 1048–1054, 1986
Liedtke AJ, Nellis SH, Mjos OD: Effects of reducing fatty acid metabolism on mechanical function in regionally ischemic hearts. Am J Physiol 247: H387-H394, 1984
Shikama H, Noshiro O, Ohta A, Ohata I: Effects of acylcarnitine transferase inhibitors on adenine nucleotide metabolism and ischemic tissue injury in isolated perfused rat hearts. Jpn Heart J 29: 723–734, 1988
Hekimian G, Feuvray D: Reduction of ischemia-induced acylcarnitine accumulation by TDGA and its influence on lactate dehydrogenase release in diabetic rat hearts. Diabetes 35: 906–910, 1986
Renstrom B, Nellis SH, Liedtke AJ: Metabolic oxidation of glucose during early myocardial reperfusion. Circ Res 65: 1094–1111, 1989
Mjos OD, Miller NE, Riemersma RA, Oliver MF: Effects of dichloroacetate on myocardial substrate extraction, epicardial ST-segment elevation, and ventricular blood flow following coronary occlusion in dogs. Cardievasc Res 10: 427–436, 1976
Mjos OD, Ichihara K, Fellenius E, Myrmel T, Neely JR: Fatty acids suppress recovery of heart function after hypothermic perfusion. Ann Thor Surg 52: 965–970, 1991
Bachmann E, Weber E: Biochemical mechanisms of oxfenicine cardiotoxicity. Pharmacology 36: 238–248, 1988
Greaves P, Martin J, Michel MC, Mompon P: Cardiac hypertrophy in the dog and rat induced by oxfenicine, an agent which modifies muscle metabolism. Arch Toxieol Suppl.7: 488–493, 1984
Litwin SE, Raya, TE, Gay RG, Bedotto JB, Bahl JJ, Anderson PG, Goldman S, Bressler R: Chronic inhibition of fatty acid oxidation: a new model of diastolic dysfunction. Am J Physiol 258: H51-H56, 1990
Reinhauer H, Adrian M, Rosen P, Schmitz F-J: Influence of carnitine acyltransferase inhibitors on the performance and metabolism of rat cardiac muscle. J Clin Chem Clin Biochem 28: 335–339, 1990
Bergman G, Atkinson L, Metcalfe J, Jackson N, Jewitt DE: Beneficial effect of enhanced carbohydrate utilisation after oxfenicine (L-hydroxyphenylglycine) in angina pectoris. Eur Heart J 1: 247–253, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hendrickson, S.C., St. Louis, J.D., Lowe, J.E. et al. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem 166, 85–94 (1997). https://doi.org/10.1023/A:1006886601825
Issue Date:
DOI: https://doi.org/10.1023/A:1006886601825