Abstract
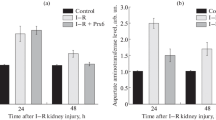
Reactive oxygen species (ROS; O2-, H2O2, and OH·), normal by-products of cellular metabolic processes, are kept in control by antioxidant enzymes, such as catalase, glutathione peroxidase (GPX) and superoxide dismutases (SODs). To understand the role of antioxidant enzymatic defenses against ROS injury following ischemia-reperfusion, we examined the effect on kidney exposed to varying periods (30, 60 or 90 min) of ischemia followed by different periods of reperfusion. The enzymatic activities and protein levels of catalase, GPX, CuZnSOD and MnSOD were relatively unaffected at 30 min of ischemia followed by 0, 2 or 24 h reperfusion. However, 60 or 90 min of ischemia followed by 0, 2 or 24 h of reperfusion resulted in a decrease in activities and protein levels which paralleled the duration of ischemic injury. MnSOD activity tended to recover towards normal during reperfusion. Examination of the mRNA levels of these antioxidant enzymes demonstrated a severe decrease in mRNA levels of catalase and GPX at a time point of minimal ischemic injury (30 min of ischemia followed by reperfusion) suggesting that loss of mRNA of catalase and GPX may be the first markers of alterations in cellular redox in ischemia-reperfusion injury. Greater loss of mRNA for catalase, GPX and CuZnSOD were observed following longer periods (60 or 90 min) of ischemia. The mRNA for MnSOD was upregulated at all time points of ischemia-reperfusion injury. Actually, the greater decrease in mRNAs for catalase, GPX and CuZnSOD in the acute phase (within 24 h) subsequently showed a further decrease in these enzyme activities in the subacute phase (72 or 120 h after ischemia). These enzyme activities in the 30 min ischemia group, but not in the 90 min group, already showed tendencies for normalization at 120 h after ischemia. To understand the molecular basis of the loss of mRNA of these antioxidant enzymes during ischemia-reperfusion injury, we examined the rate of transcription by nuclear run-on assays. The similar rates of transcription in control and kidney exposed to ischemia-reperfusion indicates that the loss of mRNA for catalase, GPX and CuZnSOD are possibly due to the increased rate of turnover of their mRNAs. These studies suggest that expression of antioxidant genes during ischemia-reperfusion are not coordinately expressed and the differential loss of antioxidant enzymes may be the contributing factor(s) towards the heterogeneous renal tissue damage as a result of ischemia-reperfusion induced oxidative stress.
Similar content being viewed by others
References
Baker GL, Corry RJ, Autor AP: Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg 202: 628–641, 1985
BonVentre JV: Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993
Johnson KJ, Weinberg JM: Postischemic renal injury due to oxygen radicals. Curr Opin Nephrol Hypertens 2: 625–635, 1993
McCord JM: Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem 26: 3511–3517, 1993
Paller MS: The cell biology of reperfusion injury in the kidney. J Invest Med 42: 632–639, 1994
Kehrer JP: Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23: 21–48, 1993
Nath KA, Paller MS: Dietary deficiency of antioxidants exacerbates ischemic injury in the rat kidney. Kidney Int 38: 1109–1117, 1990
Bayati A, Kallskog O, Wolgast M: The long-term outcome of postischaemic acute renal failure in the rat. I. A functional study after treatment with SOD and sucrose. Acta Physiol Scand 138: 25–33, 1990
Castillo M, Toledo-Peryra LH, Shapiro E, Guerra E, Prough D, Frantzis P: Protective effect of allopurinol, catalase, or superoxide dismutase in the ischemic rat liver. Trans Proc 22: 490–491, 1990
Greenwald RA: Superoxide dismutase and catalase as therapeutic agents for human diseases. Free Rad Biol Med 8: 201–209, 1990
Reilly PM, Schiller HJ, Buckley JB: Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg 161: 488–503, 1991
Senga S, Onituka A, Hirose H, Yamamoto K, Niwa K: Protective effect of liposomal encapsulated superoxide dismutase on ischemically injured liver in the rat. Trans Proc 22: 2025–2026, 1990
Shanley PF, White CW, Avraham KB, Groner Y, Burke TJ: Use of transgenic animals to study disease models: Hyperoxic lung injury and ischemic acute renal failure in ‘high SOD’ mice. Renal Fail 14: 391–394, 1992
Yoshioka T, Homma T, Meyrick B, Takeda M, Moore-Jarrett T, Kon V, Ichikanea I: Oxidants induce transcriptional activation of manganese superoxide dismutase in glomerular cells. Kidney Int 46: 405–413, 1994
Singh I, Gulati S, Orak JK, Singh AK: Expression of antioxidant enzymes in rat kidney during ischemia-reperfusion injury. Mol Cell Biochem 125: 97–104, 1993
Baudhuin P, Beaufay Y, Rahrnan LI, Sellinger OZ, Wattiaux R, Jacques P, de Duve C: Tissue fractionation studies. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alaninine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J 92: 179–184, 1964
Beutler E, Blume KG, Kaplan JC, Lö hr GW, Ramot B, Valentine WN: International Committee for Standardization in Haematology: Recommended methods for red-cell enzyme analysis. Br J Haematol 35: 331–340, 1977
Spitz DR, Oberley LW: An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 179: 8–18, 1989
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72: 248–254, 1976
Abernathy JL, Steinman HM, Hill RL: Bovine erythrocyte superoxide dismutase. Subunit structure and sequence location of the intrasubunit disulfide bond. J Biol Chem 249: 7339–7347, 1974
Simonet WS, Ness GC: Transcriptional and posttranscriptional regulation of rat hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase by thyroid hormones. J Biol Chem 263: 12448–12453, 1988
Caira F, Pacot C, Bardot O, Mariki MC, Latruffe N: Transcriptional and post-transcriptional analysis of peroxisomal protein encoding genes from rat treated with an hypolipemic agent, ciprofibrate. Effect of an intermittent treatment and influence of obesity. Biochem Pharmacol 49: 611–619, 1995
Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979
Barnard ML, Snyder SJ, Engerson TD, Turrens JF: Antioxidant enzyme status of ischemic and postischemic liver and ischemic kidney in rats. Free Rad Biol Med 15: 227–232, 1993
Sela S, Shasha SM, Marhiach E, Haj M, Kristal B, Shkolnik T: Effect of oxygen tension on activity of antioxidant enzymes and on renal function of the postischemic reperfused rat kidney. Nephron 63: 199–206, 1993
Yoshioka T, Bills T, Moore-Jarrett T, Greene HL, Burr IM, Ichikawa I: Role of intrinsic antioxidant enzymes in renal oxidant injury. Kidney Int 38: 282–288, 1990
Schiller HJ, Reilly PM, Buckley GB: Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med 21: S92–S102, 1993
Pahan K, Singh AK, Smith B, Singh I: Cytochrome P-450 IIE1 in rat liver peroxisomes: Down-regulation by ischemia-reperfusion induced oxidative stress. Free Rad Biol Med 23: 963–971, 1997
Singh I: Mammalian peroxisomes: Metabolism of oxygen and reactive oxygen species. Ann NY Acad Sci 804: 612–627, 1996
Zwacka RM, Reuter A, Pfaff E, Moll J, Gorgas K, Karasawa M, Weiher H: The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J 13: 5129–5134, 1994
Dhaunsi GS, Gulati S, Singh AK, Orak JK, Asayama K, Singh I: Demonstration of Cu-Zn superoxide dismutase in rat liver peroxisomes. Biochemical and immunochemical evidence. J Biol Chem 267: 6870–6873, 1993
Liou W, Chang LY, Geuze HJ, Straus GR, Crapo JD, Slot JW: Distribution of CuZn superoxide dismutase in rat liver. Free Rad Biol Med 14: 201–207, 1993
Muse KE, Oberby TD, Sempf JM, Oberby LW: Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochem J 26: 734–753, 1994
Singh AK, Dhaunsi GS, Asayama K, Orak JK, Singh I: Demonstration of glutathione peroxidase in rat liver peroxisomes and its intraorganellar distribution. Arch Biochem Biophys 315: 331–338, 1994
Dobyan DC, Nagle RB, Bulger RE: Acute tubular necrosis in the rat kidney following sustained hypotension: Physiologic and morphologic observations. Lab Invest 37: 411–412, 1977
Glaumann B, Glaumann H, Trump BF: Studies of cellular recovery from injury. III. Ultrastructural studies on the recovery of the pars recta of the proximal tubule (P3 segment) of the rat kidney from temporary ischemia. Virchows Arch B Cell Pathol 25: 281–308, 1977
Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG: Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2 and S3 segments. Kidney Int 14: 31–49, 1978
Kiyama S, Yoshioka T, Burr IM, Kon V, Fogo A, Ichikawa I: Strategic locus for the activation of the superoxide dismutase gene in the nephron. Kidney Int 47: 536–546, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dobashi, K., Ghosh, B., Orak, J. et al. Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol Cell Biochem 205, 1–11 (2000). https://doi.org/10.1023/A:1007047505107
Issue Date:
DOI: https://doi.org/10.1023/A:1007047505107