Abstract
Purpose. To analyze the role of the kinetics of glycyrrhizic acid (GD) in its toxicity. A physiologically-based pharmacokinetic (PBPK) model that has been developed for humans.
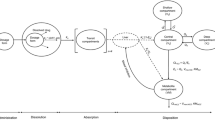
Methods. The kinetics of GD, which is absorbed as glycyrrhetic acid (GA), were described by a human PBPK model, which is based on a rat model. After rat to human extrapolation, the model was validated on plasma concentration data after ingestion of GA and GD solutions or licorice confectionery, and an additional data derived from the literature. Observed interindividual variability in kinetics was quantified by deriving an optimal set of parameters for each individual.
Results. The a-priori defined model successfully forecasted GA kinetics in humans, which is characterized by a second absorption peak in the terminal elimination phase. This peak is subscribed to enterohepatic cycling of GA metabolites. The optimized model explained most of the interindividual variance, observed in the clinical study, and adequately described data from the literature.
Conclusions. Preclinical information on GD kinetics could be incorporated in the human PBPK model. Model simulations demonstrate that especially in subjects with prolonged gastrointestinal residence times, GA may accumulate after repeated licorice consumption, thus increasing the health risk of this specific subgroup of individuals.
Similar content being viewed by others
REFERENCES
T. G. van Rossum, A. G. Vulto, R. A. deMan, J. T. Brouwer, and S. W. Schalm. Review article: Glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment. Pharmacol. Ther. 12:199–205 (1998).
F. C. Stormer, R. Reistad, and J. Alexander. Glycyrrhizic acid in liquorice—evaluation of health hazard. Food Addit. Contam. 31: 303–312 (1993).
J. W. Funder, P. T. Pearce, R. Smith, and A. I. Smith. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242:583–585 (1988).
H. J. Clewell, 3rd and B. M. Jarnot. Incorporation of pharmacokinetics in noncancer risk assessment: Example with chloropentafluorobenzene. Risk Anal. 14:265–276 (1994).
M. E. Andersen. Development of physiologically based pharmacokinetic and physiologically based pharmacodynamic models for applications in toxicology and risk assessment. Toxicol. Lett. 79: 35–44 (1995).
M. E. Andersen, H. J. Clewell, 3rd, and C. B. Frederick. Applying simulation modeling to problems in toxicology and risk assessment—a short perspective. Toxicol. Appl. Pharmacol. 133: 181–187 (1995).
B. A. Ploeger, J. Meulenbelt, and J. DeJongh. Physiologically based pharmacokinetic modeling of glycyrrhizic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Toxicol. Appl. Pharmacol. 162:177–188 (2000).
A. J. Coupe, S. S. Davis, and I. R. Wilding. Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm. Res. 8:360–364 (1991).
F. N. Christensen, S. S. Davis, J. G. Hardy, M. J. Taylor, D. R. Whalley, and C. G. Wilson. The use of gamma scintigraphy to follow the gastrointestinal transit of pharmaceutical formulations. J. Pharm. Pharmacol. 37:91–95 (1985).
J. E. Devereux, J. M. Newton, and M. B. Short. The influence of density on the gastrointestinal transit of pellets. J. Pharm. Pharmacol. 42:500–501 (1990).
D. A. Adkin, S. S. Davis, R. A. Sparrow, P. D. Huckle, A. J. Phillips, and I. R. Wilding. The effects of pharmaceutical excipients on small intestinal transit. Br. J. Clin. Pharmacol. 39:381–387 (1995).
M. Lawson, G. T. Everson, W. Klingensmith, and F. Kern, Jr. Coordination of gastric and gallbladder emptying after ingestion of a regular meal. Gastroenterology 85:866–870 (1983).
M. Proano, M. Camilleri, S. F. Phillips, M. L. Brown, and G. M. Thomforde. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am. J. Physiol. 258:G856–G862 (1990).
T. Akao. Hydrolysis of glycyrrhetyl mono-glucuronide to glycyrrhetic acid by glycyrrhetyl mono-glucuronide beta-Dglucuronidase of Eubacterium sp. GLH. Biol. Pharm. Bull. 20: 1245–1249 (1997).
K. Niinuma, Y. Kato, H. Suzuki, C. A. Tyson, V. Weizer, J. E. Dabbs, R. Froehlich, C. E. Green, and Y. Sugiyama. Primary active transport of organic anions on bile canalicular membrane in humans. Am. J. Physiol. 276:G1153–G1164 (1999).
S. Ishida, T. Ichikawa, and Y. Sakiya. Binding of glycyrrhetinic acid to rat plasma, rat serum albumin, humanserum, and human serum albumin. Chem. Pharm. Bull. 36:440–443 (1988).
R. L. Dedrick. Animal scale-up. J. Pharmacokinet. Biopharm. 1: 435–461 (1973).
M. V. Evans, W. D. Crank, H. M. Yang, and J. E. Simmons. Applications of sensitivity analysis to a physiologically based pharmacokinetic model for carbon tetrachloride in rats. Toxicol. Appl. Pharmacol. 128:36–44 (1994).
P. Armitage and G. Berry, Statistical methods in medical research, Blackwell Science Ltd., Oxford, United Kingdom, 1994.
D. Horter and J. B. Dressman. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 25:3–14 (1997).
S. Krahenbuhl, F. Hasler, B. M. Frey, F. J. Frey, R. Brenneisen, and R. Krapf. Kinetics and dynamics of orally administered 18 beta-glycyrrhetinic acid in humans. J. Clin. Endocrinol. Metab. 78:581–585 (1994).
S. Gunnarsdottir and T. Johannesson. Glycyrrhetic acid in human blood after ingestion of glycyrrhizic acid in licorice. Pharmacol. Toxicol. 81:300–302 (1997).
K. Abe, A. Suzuki, H. Katayama, Y. Tatsumi, and E. Yumioka. Determination of 18 beta-glycyrrhetic acid in human plasma by high-performance liquid chromatography. J. Chromatogr. B. Biomed. Appl. 653:112–115 (1994).
S. Takeda, H. Ono, Y. Wakui, A. Asami, Y. Matsuzaki, H. Sasaki, M. Aburada, and E. Hosoya. Determination of glycyrrhetic acid in human serum by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B. Biomed. Appl. 530:447–451 (1990).
J. Blanchard, L. M. Tang, and M. E. Earle. Reevaluation of the absorption of carbenoxolone using an in situ rat intestinal technique. J. Pharm. Sci. 79:411–414 (1990).
K. W. Heaton and L. O'Donnell. An office guide to whole-gut transit time. Patients' recollection of their stool form. J. Clin. Gastroenterol. 19:28–30 (1994).
R. Best and B. R. Walker. Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11 beta-hydroxysteroid dehydrogenase in vivo. Clin. Endocrinol. 47:231–236 (1998).
R. Brown, J. Foran, S. Olin, and D. Robinson. Physiological parameter values for PBPK models, International Life Sciences Institute and Risk Science Institute, Washington DC, 1994.
International Commission of Radiological Protection (ICRP), Publication 23: Report of the Task Group on Reference Man, Pergamon Press, New York, 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ploeger, B., Mensinga, T., Sips, A. et al. A Human Physiologically-Based Model for Glycyrrhzic Acid, A Compound Subject to Presystemic Metabolism and Enterohepatic Cycling. Pharm Res 17, 1516–1525 (2000). https://doi.org/10.1023/A:1007661209921
Issue Date:
DOI: https://doi.org/10.1023/A:1007661209921