Abstract
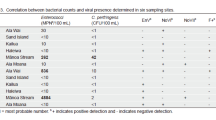
Microbiological water quality at beaches is typically measured only by indicator bacteria, even though viruses are also a concern, because classical culture-based virus assays are not suitable. In this study, molecular-based assays for the detection of enteroviruses by reverse transcriptase polymerase chain reaction (RT-PCR) were performed on 50 coastal seawater samples taken from Santa Monica Bay, CA, over a six-year period, and compared with indicator bacteria. Sample sites were near freshwater outlets in the Bay and popular sandy beaches. RT-PCR is a primer-based molecular biology technique, used to detect the genomes of specific groups or types of viruses based upon conserved sequences. Results of the 50 analyses showed our ultrafiltration concentration methods and RT-PCR protocol could be used consistently to detect enteroviruses from 20 l samples of coastal seawater. Of the 50 samples, 16 (32%) were positive for enteroviruses, 27 (54%) were negative and 7 (14%) were inconclusive. There was no significant correlation between the presence of enteroviruses and individual standard microbiological indicators of fecal contamination, specifically total coliforms, fecal coliforms, or enterococci (r=0.14, r=0.28 and r=0.34, respectively, p>0.05). However, there was a significant correlation (r=0.71) to a combined set of bacterial water quality standards, involving all three indicators, recently adopted in California. There was no significant correlation between the RT-PCR results and levels of rainfall (a large source of runoff), but our analyses demonstrated that positive results for enteroviruses were significantly more likely during the winter `wet' season than during the summer `dry' season. Our results demonstrate that RT-PCR is an effective method for the detection of enteroviruses in coastal seawater, and they suggest that bacterial indicators are not necessarily good predictors of the presence of such viruses. Enteroviruses are known to be important etiological agents of disease from recreational water contact, so analysis for their presence might be advisable at certain locations (e.g. high-use sandy beaches) or during certain seasons of the year.
Similar content being viewed by others
References
Ansari, S. A., V. S. Springthorpe & S. A. Sattar, 1991. Survival and vehicular spread of human rotaviruses-possible relation to seasonality of outbreaks. Rev. Infect. Dis. 13: 448–461.
American Public Health Association (APHA), 1992. Standard Methods for the Examination ofWater andWastewater, 17th edn. Washington, D.C.
Beril, C., J. M. Crance, F. LeGuyader, V. Apairemarchais, F. Leveque, M. Albert, M. A. Goraguer, L. Schwartzbrod, & S. Billaudel, 1996. Study of viral and bacterial indicators in cockles and mussels. Marine Poll. Bull. 32: 404–409.
Bitton, G., 1978. Indicator bacteria. In: Mitchell, R. (ed.), Water Pollution Microbiology. R. John Wiley & Sons, Inc. New York, N.Y., U.S.A.
Byrd, J. J., H.-S. Xu & R. R. Colwell, 1991. Viable but Nonculturable Bacteria in Drinking Water. Apl. Envir. Microbiol. 57: 875–878.
Cabelli, V., 1983. Health effects criteria for marine recreational water. EPA. 600/1-84-004: 7 pp.
Cabelli, V. J., A. P. Dufour, M. A. Levin & L. J. McCabe, 1982. Swimming-associated gastroenteritis and water quality. Am. J. Epidemiol. 115: 606–616.
Chamberlain, C. E. & R. Mitchell, 1978. A decay model for enteric bacteria in natural waters. In Mitchell, R. (ed.), Water Pollution Microbiology. Wiley, New York: pp. 325–348.
DeLeon, R., Y. S. C. Shieh, R. S. Baric & M. Sobsey, 1990. Detection of enteroviruses and hepatitis A virus in environmental samples by gene probes and polymerase chain reaction, Advances in water analysis and treatment. Proceedings of the Water Quality Technology Conference. American Water Works Association, San Diego, CA. 833–853.
Dubois, E., F. LeGuyader, L. Haugarreau, H. Kopecka, M. Cormier, & M. Pommepuy, 1997. Molecular epidemiological survey or rotaviruses in sewage by reverse transcriptase seminested PCR and restriction fragment length polymorphism assay. Apl. envir. Microbiol. 63: 1794–1800.
Elliott, E. L. & R. R. Colwell. 1985. Indicator organisms for estuarine and marine waters. FEMS Microbiol Rev. 32: 61–79.
Finch, G. R. & N. Fairbairn, 1991. Comparative inactivation of Poliovirus Type 3 and MS2 Coliphage in demand-free phosphate buffer by using ozone. Apl. envir. Microbiol. 57: 3121–3126.
Fleisher, J. M., 1990. The effects of measurement error on previously reported mathematical relationships between indicator organism density and swimming-associated illness: A quantitative estimate of the resulting bias. International J. Epidemiol. 19: 1100–1106.
Fleisher, J. M., D. Kay, M. Wyer & H. Merrett, 1996. The enterovirus test in the assessment of recreational water-associated gastroenteritis. Water Res. 30: 2341–2346.
Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature (London). 399: 203–210.
Garcia-Lara, J., P. Menon, P. Servais & G. Billen, 1991. Mortality of Fecal Bacteria in Seawater. Apl. envir. Microbiol. 57: 885–888.
Gerba, C. P. & J. S. McLeod, 1976. Effects of sediment on the survival of Escherichia coli in marine waters. Apl. envir. Microbiol. 32: 114–120.
Ghoul, M., T. Bernard & M. Cormier, 1986. Evidence that Escherichia coli accumulates glycine betaine from marine sediment. Apl. envir. Microbiol. 56: 551–554.
Goyal, S. M., W. N. Adams, M. L. O'Malley & D. W. Lear, 1984. Human pathogenic viruses at sewage sludge disposal sites in the Middle Atlantic region. Apl. envir. Microbiol. 48: 757–763.
Griffin, D. W., C. J. Gibson, III, E. K. Lipp, K. Riley, J. H. Paul, & J. B. Rose. 1999. Detection of viral pathogens by Reverse Transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Apl. envir. Microbiol. 65: 4118–4125.
Haile, R. W., J. S. Witte, M. Gold, R. Cressey, C. McGee, R. C. Millikan, A. Glasser, N. Harawa, C. Ervin, P. Harmon, J. Harper, J. Dermand, J. Alamillo, K. Barrett, M. Nides, & G.-Y. Wang, 1999. The health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology 10: 355–363.
Hughes, M. S., P. V. Coyle & J. H. Connolly, 1992. Enteroviruses in recreational waters of Northern Ireland. Epidemiol Infect. 108: 529–536.
Hughes, M. S., E. M. Hoey & P. V. Coyle, 1993. A nucleotide sequence comparison of Coxsackievirus B4 isolates from aquatic samples and clinical specimens. Epidemiol Infect. 110: 389–398.
Jiang, S., R. T. Noble & W. Chu, 2001. Human Adenoviruses and coliphages in urban runoff-impacted coastal waters of southern California. Apl. envir. Microbiol. 67: 179–184.
Kapikian, A. Z., 1993. Viral gastroenteritis. J. am. Med. Ass. 269: 627–630.
Kay, D., J. M. Fleisher, A. F. Godfree, F. Jones, R. L. Salmon, R. Shore, M. D. Wyer & R. Zelenauch-Jacquotte, 1994. Predicting likelihood of gastroenteritis from sea bathing: Results from randomised exposure. Lancet 344: 905–909.
Kopecka, H., S. Dubrou, J. Prevot, J. Marechal & J. M. Lopez-Pila, 1993. Detection of naturally occurring enteroviruses in waters by reverse transcription polymerase chain reaction and hybridization. Apl. envir. Microbiol. 59: 1213–1219.
LeGuyader, F., V. Apaire-Marchais, J. Brillet & S. Billaudel, 1993. Use of genomic probes to detect hepatitis A virus and enterovirus RNAs in wild shellfish and relationship of viral contamination to bacterial contamination. Apl. envir. Microbiol. 59: 3963–3968.
McNeill, A. R., 1992. Recreational water quality. In D.W.C. & D.W.H. (eds), Pollution of Tropical Aquatic Systems. CRC Press Inc., Boca Raton, FL: 193–216.
Moore, A. C., G. F. Herwaldt, G. F. Craun, R. L. Calderon, A. K. Highsmith & D. D. Juranek, 1994. Waterborne disease in the United States, in 1991 and 1992. Am. Water Works Ass. J. 86: 87–99.
Muir, P., U. Kammerer, K. Korn, M. N. Mulers, T. Poyry, B. Weissbrich, R. Kandolf, G. M. Cleator & A. M. Van Loon, 1998. Molecular typing of enteroviruses: Current status and future requirements. Clin. Microbiol. Rev. 11: 202–227.
Noble, R. T. & J. A. Fuhrman, 1997. Virus decay and its causes in coastal waters. Apl. Envir. Microbiol. 63: 77–83.
Perez-Rosas, N. & T. C. Hazen, 1988. In situ survival of Vibrio cholerae and Escherichia coli in tropical coral reefs. Apl. envir. Microbiol. 54: 1–9.
Pichard, S. L. & J. H. Paul, 1991. Detection of gene expression in genetically engineered microorganisms and natural phytoplankton populations in the marine environment by messenger RNA analysis. Apl. envir. Microbiol. 57: 1721–1727.
Piña, S., M. Puig, F. Lucena, J. Jofre & R. Girones, 1998. Viral pollution in the environment and in shellfish: Human adenovirus detection by PCR as an index of human viruses. Apl. envir. Microbiol. 64: 3376–3382. 184
Reynolds, K. A., K. Roll, R. S. Fujioka, C. P. Gerba & I. L. Pepper, 1998. Incidence of enteroviruses in Mamala Bay, Hawaii using cell culture and direct polymerase chain reaction methodologies. Can. J. Microbiol. 44: 598–604.
Rose, J. B., X. T. Zhou, D. W. Griffin & J. H. Paul, 1997. Comparison of PCR and plaque assay for detection and enumeration of coliphage in polluted marine waters. Apl. envir. Microbiol. 63: 4564–4566.
Santa Monica Bay Restoration Project (SMBRP) 1994. Public summary of the Santa Monica Bay Restoration Plan. Georges and Shapiro. Sacramento, CA.
Seyfried, P. L., R. S. Tobin, N. E. Brown & P. F. Ness, 1985a. A prospective study of swimming-related illness. II. Morbidity and the microbiological quality of water. Am. J. Public Health 75: 1071 pp.
Seyfried, P. L., R. S. Tobin, N. E. Brown & P. F. Ness, 1985b. A prospective study of swimming-related illness. I. Swimmingassociated health risks. Am. J. Public Health 75: 1068 pp.
Suttle, C. A., A. M. Chan & M. T. Cottrell, 1991. Use of ultra-filtration to isolate viruses from seawater which are pathogens of marine phytoplankton. Apl. envir. Microbiol. 57: 721–726.
Tsai, Y., M. D. Sobsey, L. R. Sangermano & C. J. Palmer, 1993. Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase polymerase chain reaction. Apl. envir. Microbiol. 59: 3488–3491.
Yamashita, T., K. Sakae, Y. Ishihara & S. Isomura, 1992. A 2-year survey of the prevalence of enteric viral infections in children compared with contamination in locally harvested oysters. Epidemiol. Infect. 108: 155–163.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Noble, R.T., Fuhrman, J.A. Enteroviruses detected by reverse transcriptase polymerase chain reaction from the coastal waters of Santa Monica Bay, California: low correlation to bacterial indicator levels. Hydrobiologia 460, 175–184 (2001). https://doi.org/10.1023/A:1013121416891
Issue Date:
DOI: https://doi.org/10.1023/A:1013121416891