Abstract
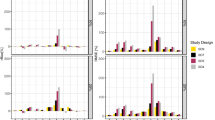
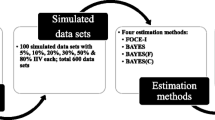
The aim of this study was to assess the type I error rate when applying the likelihood ratio (LR) test, for components of the statistical sub-model in NONMEM. Data were simulated from a pharmacokinetic one compartment intravenous bolus model. Two models were fitted to the data, the simulation model and a model containing one additional parameter, and the difference in objective function values between models was calculated. The additional parameter was either (i) a covariate effect on the interindividual variability in CL or V, (ii) a covariate effect on the residual error variability, (iii) a covariance term between CL and V, or (iv) interindividual variability in V. Factors in the simulation conditions (number of individuals and samples per individual, interindividual and residual error magnitude, residual error model) were varied systematically to assess their potential influence on the type I error rate. Different estimation methods within NONMEM were tried. When the first-order conditional estimation method with interaction (FOCE INTER) was used the estimated type I error rates for inclusion of a covariate effect (i) on the interindividual variability, or (ii) on the residual error variability, were in agreement with the type I error rate expected under the assumption that the model approximations made by the estimation method are negligible. When the residual error variability was increased, the type I error rates for (iii) inclusion of covariance between ηCL–ηV were inflated if the underlying residual distribution was lognormal, or if a normal distribution was combined with too little information in the data (too few samples per subject or sampling at uninformative time-points). For inclusion of (iv)ηV, the type I error rates were affected by the underlying residual error distribution; with a normal distribution the estimated type I error rates were close to the expected, while if a non-normal distribution was used the type I errors rates increased with increasing residual variability. When the first-order (FO) estimation method was used the estimated type I error rates were higher than the expected in most situations. For the FOCE INTER method, but not the FO method, the LR test is appropriate when the underlying assumptions of normality of residuals, and of enough information in the data, hold true. Deviations from these assumptions may lead to inflated type I error rates.
Similar content being viewed by others

REFERENCES
E. I. Ette and T. M. Ludden. Population pharmacokinetic modeling: the importance of informative graphics. Pharm. Res. 12:1845-1855 (1995).
E. I. Ette. Statistical graphics in pharmacokinetics and pharmacodynamics: a tutorial. Ann Pharmacother. 32:818-828 (1998).
S. L. Beal and L. B. Sheiner (eds.). NONMEM Users Guides. University of California, San Francisco, CA, 1992.
U. Wahlby, E. N. Jonsson, and M. O. Karlsson. Assessment of actual significance levels for covariate effects in NONMEM. J. Pharmacokinet. Pharmacodyn. 28:231-252 (2001).
J. V. S. Gobburu and J. Lawrence. Application of resampling techniques to estimate exact significance levels for covariate selection during nonlinear mixed effects model building: some inferences. Pharm. Res. 19:92-98 (2002).
S. L. Beal and L. B. Sheiner. Estimating population kinetics. Crit. Rev. Biomed. Eng. 8:195-222 (1982).
M. Davidian and D. M. Giltinan. Nonlinear Models for Repeated Measurement Data.Monographs on Statistics and Applied Probability 62, Chapman & Hall, London, 1995.
S. L. Beal. Population pharmacokinetic data and parameter estimation based on their first two statistical moments. Drug Met. Rev. 15:173-193 (1984).
S. L. Beal and L. B. Sheiner (eds.). NONMEM Users Guide-Part VII. University of California, San Francisco, CA, 1992.
D. O. Stram and J. W. Lee. Variance components testing in the longitudinal mixed effects model. Biometrics 50:1171-1177 (1994).
J. C. Pinheiro and D. M. Bates. Mixed-Effects Models in S and S-PLUS. Statistics and Computing, Springer, New York, 2000.
E. F. Vonesh, V. M. Chinchilli, and K. Pu. Goodness-of-fit in generalized nonlinear mixedeffects models. Biometrics 52:572-587 (1996).
G. E. P. Box. Non-normality and tests on variances. Biometrika 40:318-335 (1953).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wählby, U., Bouw, M.R., Jonsson, E.N. et al. Assessment of Type I Error Rates for the Statistical Sub-model in NONMEM. J Pharmacokinet Pharmacodyn 29, 251–269 (2002). https://doi.org/10.1023/A:1020254823597
Issue Date:
DOI: https://doi.org/10.1023/A:1020254823597