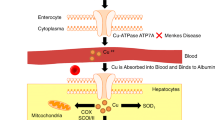
Abstract
Relevant biological effects associated with mild to moderate copper deficiency and copper excess are unknown. It is difficult to identify markers of these early changes because limits of the homeostatic range are still undefined and early changes may represent adaptive responses that do not imply necessarily risk of damage. We report here a series of studies carried out to shed light on the responses within the homeostatic range, by assessing classic parameters of copper status in humans at different copper exposure. In adult healthy volunteers that had an estimated daily intake of 0.9 mg Cu/day (approximately 15 μg/kg/d), exposure to additional 50–60 μg of copper/kg/day for three months or up to 150 μg/kg/d for two months resulted in no significant changes of SOD activity in erythrocytes, of copper concentration (in serum, erythrocytes and mononuclear cells) and of serum ceruloplasmin (ANOVA). Neither were found differences by gender or age. As in previous studies in infants, the non-ceruloplasmin copper fraction was positively correlated to serum copper (r=0.58). Assessing variations on copper absorption, infants supplemented/not supplemented with oral copper (80 ug/kg/14 days), at age 1 and 3 months, showed copper absorption close to 80% at both ages; no effect was observed for age or supplementation, suggesting that either these concentrations do not elicit regulatory mechanisms or that at this age down regulation for copper absorption is not efficient. These studies indicate that in the range of the copper homeostasis area the markers tested are not suitable to detect mild changes (within the homeostatic range) of copper metabolism.
Similar content being viewed by others
References
Brewer GJ. 1998 Wilson disease and canine copper toxicosis. Am J Clin Nutr 67, 1087S-1090S.
Cartwright GE. 1959 Copper metabolism in human subjects. In: McElroy WD, Glass B, eds. Copper Metabolism. Baltimore: John Hopkins Press; 274-310.
Cordano A. 1998 Clinical manifestations of nutritional copper defi-ciency in infants and children. Am J Clin Nutr 67, 1012S-1016S.
Cordano A, Baertl JM, Graham G. 1964 Copper deficiency in infancy. Pediatrics 34, 324-246.
Danks DM. 1988 Copper deficiency in humans. Ann Rev Nutr 8, 235-257.
Eife R, Weiss M, Müller-Hocker J, Lang T, Barros V, Sigmund B, Thanner F, Welling P, Lange H, Wolf W, Rodeck B, Kittel J, Schramel P, Reiter K. 1999 Chronic poisoning by copper in tap water: II. Copper intoxication with predominantly systemic symptoms. Eur J Med Res 4, 224-228.
Food and Nutrition Board, Institute of Medicine 2001 Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press.
IPCS 1998 Environmental Health Criteria 200. Copper. WHO: Geneva.
Kaler SG. 1999 Diagnosis and therapy of Menkes syndrome, a genetic form of copper deficiency. Am J Clin Nutr 67, 1029S-1034S.
Mercer JFB, Grimes A, Ambrosini L et al.. 1994 Mutations in the murine homologue of the Menkes disease gene in dappled and blotchy mice. Nat Genet 6, 374-378.
Milne DB. 1999 Copper intake and assessment of copper status. Am J Clin Nutr 67, 1041S-1045S.
Milne DB, Klevay LM, Hunt JR. 1988 Effects of ascorbic acid supplements and a diet marginal in copper on indices of copper nutriture in women. Nutr Res 8, 865-873.
Milne DB, Johnson PE, Klevay LM, Sandstead H. 1990 Effect of copper intake on balance, absorption, and status indices of copper in man. Nutr Res 10, 975-986.
Olivares M, Pizarro F, Speisky H, Lönnerdal B, Uauy R. 1998 Copper in infant nutrition: safety of WHO provisional guideline value for copper content of drinking water. J Pediatr Gastroenterol Nutr 26, 251-257.
Olivares M, Araya M, Uauy R. 2000 Copper homeostasis in infant nutrition: deficit and excess. J Pediatr Gastroenterol Nutr 31, 102-111.
Olivares M, Lönnerdal B, Abrams SA, Pizarro F, Uauy R. 2002 Effects of age and copper intake on copper absorption in young infants measured using 65Cu as a tracer. Am J Clin Nutr in press.
Peña MMO, Lee J, Thiele DJ. 1999 A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 129, 1251-1260.
Pizarro F, Olivares M, Uauy R, Contreras P, Rebelo A, Gidi V. 1999 Acute gastrointestinal effects of graded levels of copper in drinking water. Environ Health Perspect 107, 117-121.
Reiser S, Smith JC Jr, Mertz W, Holbrook JT, Scholfield DJ, Powell AS, Canfield WK, Canary JJ. 1985 Indices of copper status in humans consuming a typical American diet containing either fructose or starch. Am J Clin Nutr 42, 242-251.
Scheinberg IH, Gitlin D. 1984 Wilson's Disease. Philadelphia: WB Saunders.
Scheinberg IH, Sternlieb I. 1996 Wilson disease and copper toxicosis. Am J Clin Nutr 63, 842S-845S.
Schilsky ML, Sternlieb I. 1993 Animal models of copper toxicosis. Adv Vet Sci Comp Med 37, 357-377.
Turnlund JR, Keyes WR, Anderson HL, Accord LL. 1989 Copper absorption and retention in young men at three levels of dietary copper by use of stable isotope 65Cu. Am J Clin Nutr 49, 870-878.
Turnlund JR, Keen CL, Smith RG. 1990 Copper status and urinary and salivary copper in young men at three levels of dietary copper. Am J Clin Nutr 51, 658-664.
Uauy R, Castillo-Duran C, Fisberg M, Fernandez N, Valenzuela A. 1985 Red cell superoxide dismutase activity as an index of human copper nutrition. J Nutr 115, 1650-1655.
Van de Sluis, Breen M, Nanjir M et al. 1999 Genetic mapping of the copper toxicosis locus in Bedlington terriers with copper toxicosis. Hum Mol Genet 8, 501-507.
Wilson SAK. 1912 Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain 34, 295-509.
Yamaguchi Y, Heiny ME, Shimizu N, Aoki T, Gitlin GD. 1994 Expression of the Wilson disease gene is deficient in the Long-Evans Cinnamon rat. Biochem J 301, 1-4.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Araya, M., Olivares, M., Pizarro, F. et al. Copper exposure and potential biomarkers of copper metabolism. Biometals 16, 199–204 (2003). https://doi.org/10.1023/A:1020723117584
Issue Date:
DOI: https://doi.org/10.1023/A:1020723117584