Abstract
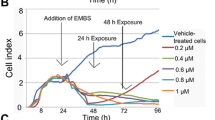
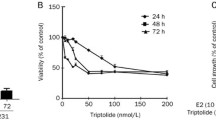
2-Methoxyoestradiol (2-MeOE2) is an endogenous oestrogen metabolite which inhibits tubulin polymerisation and has anti-tumour and anti-angiogenic activity. 2-MeOE2 induces apoptosis in a wide range of cancer cell types and has recently been demonstrated to cooperate with TRAIL to induce apoptosis in breast cancer cells. 2-Methoxyoestradiol-3,17-bis-O,O-sulphamate (2-MeOE2bisMATE) is a sulfamoylated derivative of 2-MeOE2 with enhanced activity and improved pharmacokinetic properties, and 2-MeOE2bisMATE is a promising candidate for early clinical trials. It is important, therefore, to understand the mechanisms by which 2-MeOE2bisMATE acts, and whether it retains the ability to cooperate with TRAIL. We demonstrate that 2-MeOE2bisMATE-induced apoptosis of CAL51 breast cancer cells was associated with rapid activation of caspase 3 and 9, but not caspase 8 (as measured by BID cleavage) and was completely prevented by the caspase inhibitor zVADfmk. Interfering with Fas- or TRAIL-receptor function did not prevent 2-MeOE2bisMATE-induced apoptosis. Whereas CAL51 cells were resistant to TRAIL-induced apoptosis, 2-MeOE2bisMATE and TRAIL cooperated to induce cell death. This apoptosis was associated with enhanced activation of caspases, but not increased expression of the DR5 TRAIL receptor, previously demonstrated to be induced by 2-MeOE2. Therefore, 2-MeOE2bisMATE-induced apoptosis is dependent on caspases and like 2-MeOE2, 2-MeOE2bisMATE can overcome resistance to TRAIL by stimulating activation of downstream caspases. Our results suggest that 2-MeOE2bisMATE and TRAIL might be a particularly effective combination of anti-cancer agents.
Similar content being viewed by others
References
Stennicke HR, Salvesen GS. Caspases—Controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta 2000; 1477: 299–306.
Kroemer G. Mitochondrial control of apoptosis: An introduction. Biochem Biophys Res Commun 2003; 304: 433–435.
Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. Journal of Cell Physiol 2003; 195: 158–167.
Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science 1998: 1305–1308.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501.
D’Amato RJ, Lin CM, Flynn E, Folman J, Hamel E. 2–Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicines site. Proc Nat. Acad Sci USA 1994; 91: 3964–3968.
Sattler M, Quinnan LR, Price YB, et al. 2–Methoxyestradiol alters cell motility, migration, and adhesion. Blood 2003; 102: 289–296.
Yue TL, Wang X, Louden CS, et al. 2–Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: Possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol Pharmacol 1997; 51: 951–962.
Reiser F, Way D, Bernas M, Witte M, Witte C. Inhibition of normal and experimental angiotumour endothelial cell proliferation and cell cyle progression by 2–methoxyestradiol. Proc Soc Exp Biol Med 1998; 219: 211–216.
Chauhan D, Catley L, Hideshima T, et al. 2–Methoxyestradiol overcomes drug resistancein multiple myeolma cells. Blood 2002; 100: 2187–2194.
MacCarthy-Morrogh L, Townsend PA, Purohit A, et al. G. Differential effects of estrone and estrone-3–O-sulfamate derivatives on mitotic. Arrest, apoptosis, and microtubule assembly in human breast cancer cells. Cancer Res 2000; 60: 5441–5450.
Mabjeesh NJ, Escuin D, La Vallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 2003; 3: 363–375.
Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2–methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 1994; 368: 237–239.
Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2–methoxyestradiol and taxol. Cancer Res 1997; 57: 81–86.
Yue TL, Wang X, Louden CS, et al. 2–Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: Possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol Pharmacol 1997; 51: 951–962.
LaValle TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2–Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res 2002; 62: 3691–3697.
Mukhopadhyay T, Roth JA. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene 1997; 14: 379–384.
Seegers JC, Lottering ML, Grobler CJ, et al. The mammalian metabolite, 2–methoxyestradiol, affects P53 levels and apoptosis induction in transformed cells but not in normal cells. J Steroid Biochem Mol Biol 1997; 62: 253–267.
Carothers AM, Hughes SA, Ortega D, Bertagnolli MM. 2–Methoxyestradiol induces p53–associated apoptosis of colorectal cancer cells. Cancer Lett 2002; 187: 77–86.
Vousden KH, Lu X. Live or let die: The cell’s response to p53. Nat Rev Cancer 2002; 2: 594–604.
Attalla H, Westberg JA, Andersson LC, Adlercreutz H, Makela TP. 2–Methoxyestradiol-induced phosphorylation of Bcl-2: Uncoupling from JNK/SAPK activation. Biochem Biophys Res Commun 1998; 247: 616–619.
Basu A, Halder S. Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxl-or 2–methoxyestradiol-induced apoptosis. FEBS Lett 2003; 538: 41–47.
Kumar AP, Garcia GE, Orsborn J, Levin VA, Slaga TJ. 2–Methoxyestradiol interferes with NF kappa B transcriptional activity in primitive neuroectodermal brain tumours: Implications for management. Carcinogenesis 2003; 24: 209–216.
Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappa B-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA 1999; 96: 9136–9141.
Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappa B that blocks TN Falpha-induced apoptosis. Genes Dev 1999; 13: 382–387.
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000; 407: 390–395.
Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukaemia cell and is role in cellular sensitivity to ROS-generating anticancer agents. Blood 2003; 101: 4098–4104.
Kachadourian R, Liochev SI, Cabelli D, Patel MN, Fridovich I, Day BJ. 2–Methoxyestradiol does not inhibit superoxide dismutase. Arch Biochem Biophys 2001; 392: 349–353.
LaValle TM, Zhan XH, Johnson MS, et al. 2–Methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res 2003; 63: 468–475.
Purohit A, Hejaz HA, Walden L, et al. The effect of 2–methoxyestrone-3–O-sulphamate on the growth of breast cancer cells and induced mammary tumours. Int J Cancer 2000; 85: 584–589.
Day JM, Newman SP, Comninos A, et al. The effects of 2–substituted oestrogen sulphamates on the growth of prostate and ovarian cancer cells. J Steroid Biochem Mol Biol 2003; 84: 317–325.
Wood L, Leese MP, Leblond B, et al. Inhibition of superoxide dismutase by 2–methoxyoestradiol analogues and oestrogen derivatives: Structure-activity relationships. Anticancer Drug Design 2001; 16: 209–215.
Raobaikady B, Purohit A, Chander SK, et al. Inhibition of MCF-7 breast cancer cell proliferation and in vivo steroid sulphatase activity by 2–methoxyoestradiol-bis-sulphamate. J Steroid Biochem Mol Biol 2003; 84: 351–358.
Newman SP, Leese MP, Purohit A, et al. Inhibition of in vitro angiogenesis by 2–methoxy and 2–ethyl-estrogen sulfamates. Int J Cancer, In press.
Suzuki RN, Newman SP, Purohit A, et al. Growth inhibition of multi-drug-resistant breast cancer cells by 2–methoxyoestradiol-bis-sulphamate and 2–ethyloestradiol-bis-sulphamate. Journal of Steroid Biochemistry & Molecular Biology 2003; 84: 269–278.
Ireson CR, Chander SK, Purohit A, et al. Pharamacokinetics and efficacy of 2–methoxyoestradiol and 2–methoxyoestradiol and 2–methoxyoestradiol sulphamate in vivo in rodents. Br J Cancer, In press.
Gioanni J, Le Francois D, Zanghellini E, et al. Establishment and characterization of a new tumorigenic cell line with a normal karyotype derived from a human breast adenocarcinoma. Br J Cancer 1990; 62: 8–13.
Brimmell M, Burns JS, Munson P, et al. High level expression of differentially localized BAG-1 isoforms in some oestrogen receptor-positive human breast cancers. Br J Cancer 1999; 81: 1042–1051.
Ariumi Y, Ueda K, Masutani M, et al. In vivo phosphorylation of poly (ADP-ribose) polymerase is independent of its activation. FEBS Letters 1998; 436: 288–292.
Germain M, Affar EB, D’Amours D, Dixit VM, Salvesen GS, Poirier GC. Journal of Biological Chemistry 1999; 274: 28379–28384.
Mouzakiti A, Packham G. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Burkitt’s lymphoma cell lines. Br J Haematol 2003; 122: 61–69.
Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Letters 1999; 442: 117–121.
Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 1999; 19: 8469–8478.
Chauhan D, Catley L, Hideshima T, et al. 2–methoxyestradiol overcomes drug resistance in multiple myeloma cells. Blood 2002; 100: 2187–2194.
Newton K, Harris AW, Batch ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORTI enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J 1998; 17: 706–718.
Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of fas-associated death domain protein in cell cycle progression localized to a single amino acid and its C-terminal region. Immunity 2003; 18: 513–521.
Walsh CM, Wen BG, Chainnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity 1998; 8: 439–449.
Zornig M, Hueber AO, Evan G. p53–dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr Biol 1998; 8: 467–470.
Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME. Phosphorylation of FADD/MORTI at serine 194 and association with a 70–kDa cell cycle-regulated protein kinase. J Immunology 2000; 164: 1236–1242.
Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 lignd. J Clin Invest 1999; 104: 155–162.
Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–163.
Lacour S, Micheau O, Hammann A, et al. Chemotherapy enhances TNF-related apoptosis-inducing ligand DISC assembly in HT29 human colon cancer cells. Oncogene 2003; 22: 1807–1816.
Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene 2003; 22: 2034–2044.
Bu S, Blaukat A, Fu X, Heldin NE, Landstrom M. Mechanisms for 2–methoxyestradiol-induced apoptosis of prostate cancer cells. FEBS Lett 2002; 531: 141–151.
Djavaheri-Mergny M, Wietzerbin J, Besancon F. 2–Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene 2003; 22: 2558–2567.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wood, L., Leese, M.P., Mouzakiti, A. et al. 2-MeOE2bisMATE induces caspase-dependent apoptosis in CAL51 breast cancer cells and overcomes resistance to TRAIL via cooperative activation of caspases. Apoptosis 9, 323–332 (2004). https://doi.org/10.1023/B:APPT.0000025809.80684.bd
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000025809.80684.bd