Abstract
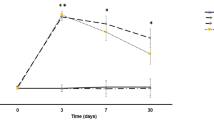
Free fatty acids (FFA) and lactic acid are markers of secondary cellular injury following traumatic brain injury (TBI). We previously showed that animals fed a creatine (Cr)-enriched diet are afforded neuroprotection following TBI. To further characterize the neuroprotective Cr diet, we studied neurochemical changes in cortex and hippocampus following a moderate injury. Adult rats were fed either a control or Cr-supplemented diet (0.5%, 1%) for 2 weeks before TBI. At 30 min or 6 h after injury, tissue was processed for quantitative analysis of neurochemical changes. Both lactate and FFA were significantly increased in all tissues ipsilateral to the injury. Cr-fed animals had significantly lower levels, although the levels were elevated compared to sham controls. Animals fed a 1% Cr-diet were afforded greater neuroprotection than animals fed a 0.5% Cr diet. These results support the idea that a Cr-enriched diet can provide substantial neuroprotection in part by suppressing secondary brain injury.
Similar content being viewed by others
references
Faden, A. I., Demediuk, P., Panter, S. S., and Vink, R. 1989. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244:798–800.
Globus, M. Y.-T., Alonso, O., Dietrich, W. D., Busto, R., and Ginsberg, M. D. 1995. Glutamate release and free radical production following brain injury: Effects of post-traumatic hypothermia. J. Neurochem. 65:1704–1711.
Gorman, L. K., Pang, K., Frick, K. M., Givens, B., and Olton, D. S. 1994. Acetylcholine release in the hippocampus: Effects of cholinergic and GABAergic compounds in the medial septal area. Neurosci. Lett. 166:199–202.
Kawamata, T., Katayama, Y., Hovda, D. A., Yoshino, A., and Becker, D. P. 1995. Lactate accumulation following concussive brain injury: The role of ionic fluxes induced by excitatory amino acids. Brain Res. 674:196–204.
Nilsson, P., Hillered, L., Ponten, U., and Ungerstedt, U. 1990. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 10:631–637.
Palmer, A. M., Marion, D. W., Botscheller, M. L., Swedlow, P. E., Styren, S. D., and DeKosky, S. T. 1993. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 61:2015–2024.
Delahunty, T. M. 1992. Mild traumatic brain injury enhances muscarinic receptor-linked inositol phosphate production in rat hippocampus. Brain Res. 594:307–310.
Dhillon, H. S., Carbary, T., Dose, J., Dempsey, R. J., and Prasad, M. R. 1995. Activation of phosphatidylinositol bisphosphate signal transduction pathway after experimental brain injury: A lipid study. Brain Res. 698:100–106.
Dhillon, H. S., Yang, L., Padmaperuma, B., Dempsey, R. J., Fiscus, R. R., and Renuka Prasad, M. 1995. Regional concentrations of cyclic nucleotides after experimental brain injury. J. Neurotrauma 12:1035–1043.
Hayes, R. L., Jenkins, L. W., and Lyeth, B. G. 1992. Neurotransmitter-mediated mechanisms of traumatic brain injury: acetylcholine and excitatory amino acids. J. Neurotrauma 9:S173–S187.
Lyeth, B. G., Gong, Q.-Z., Dhillon, H. S., and Prasad, M. R. 1996. Effects of muscarinic receptor antagonism on the phosphatidylinositol bisphosphate signal transduction pathway after experimental brain injury. Brain Res. 742:63–70.
Prasad, M. R., Dhillon, H. S., Carbary, T., Dempsey, R. J., and Scheff, S. W. 1994. Enhanced phosphodiestric breakdown of phosphatidylinositol bisphosphate after experimental brain injury. J. Neurochem. 63:773–776.
Kristian, T. and Siesjo, B. K. 1998. Calcium in ischemic cell death. Stroke 29:705–718.
Vink, R. 1993. Nuclear magnetic resonance characterization of secondary mechanisms following traumatic brain injury. Mol. Chem. Neuropathol. 18:279–297.
Prasad, M. P., Ramaiah, C., McIntosh, T. K., Dempsey, R. J., Hipkens, S., and Yurek, D. 1994. Regional levels of lactate and norepinephrine after experimental brain injury. J. Neurochem. 63:1086–1094.
Hovda, D. A., Becker, D. P., and Katayama, Y. 1992. Secondary injury and acidosis. J. Neurotrauma 9 (Suppl 1):S47–S60.
Dhillon, H. S., Donaldson, D., Dempsey, R. J., and Prasad, M. R. 1994. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J. Neurotrauma 11:405–415.
Dhillon, H. S., Dose, J. M., Scheff, S. W., and Prasad, M. R. 1997. Time course of changes in lactate and free fatty acids after experimental brain injury and relationship to morphologic damage. Exp. Neurol. 146:240–249.
Balestrino, M., Rebaudo, R., and Lunardi, G. 1999. Exogenous creatine delays anoxic depolarization and protects from hypoxic damage: Dose-effect relationship. Brain Res. 816:124–130.
Holtzman, D., Togliatti, A., Khait, I., and Jensen, F. 1998. Creatine increases survival and suppresses seizures in the hypoxic immature rat. Pediatr. Res. 44:410–414.
Klivenyi, P., Ferrante, R. J., Matthews, R. T., Bogdanov, M. B., Klein, A. M., Andreassen, O. A., Mueller, G., Wermer, M., Kaddurah-Daouk, R., and Beal, M. F. 1999. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 5:347–350.
Matthews, R. T., Yang, L., Jenkins, B. G., Ferrante, R. J., Rosen, B. R., Kaddurah-Daouk, R., and Beal, M. F. 1998. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J. Neurosci. 18:156–163.
Wilken, B., Ramirez, J. M., Probst, I., Richter, D. W., and Hanefeld, F. 1998. Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr. Res. 43:8–14.
Sullivan, P. G., Geiger, J. D., Mattson, M. P., and Scheff, S. W. 2000. Dietary supplement creatine protects against traumatic brain injury. Ann. Neurol. 48:723–729.
Baldwin, S. A. and Scheff, S. W. 1996. Intermediate filament change in astrocytes following mild cortical contusion. Glia 16:266–275.
Sullivan, P. G., Keller, J. N., Mattson, M. P., and Scheff, S. W. 1998. Traumatic brain injury alters synaptic homeostasis: Implications for impaired mitochondrial and transport function. J. Neurotrauma 15:789–798.
Baldwin, S. A., Fugaccia, I., Brown, D. R., Brown, L. V., and Scheff, S. W. 1996. Blood-brain barrier breach following cortical contusion in the rat. J. Neurosurg. 85:476–481.
Baldwin, S. A., Gibson, T., Callihan, C. T., Sullivan, P. G., Palmer, E., and Scheff, S. W. 1997. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J. Neurotrauma 14:385–398.
Scheff, S. W., Baldwin, S. A., Brown, R. W., and Kraemer, P. J. 1997. Morris water maze deficits in rats following traumatic brain injury: Lateral controlled cortical impact. J. Neurotrauma 14:615–627.
Sullivan, P. G., Bruce-Keller, A., Rabchevsky, A. G., Christakos, S., St Clair, D. K., Mattson, M. P., and Scheff, S. W. 1999. Exacerbation of damage and altered NFkB activation in mice lacking tumor necrosis factor receptors following traumatic brain injury. J. Neurosci. 19:6248–6256.
Scheff, S. W. and Sullivan, P. G. 1999. Cyclosporin A significantly ameiliorates cortical damage following experimental traumataic brain injury in rodents. J. Neurotrauma 16:783–792.
Ponten, U., Ratcheson, R. A., Salford, L. G., and Siesjo, B. K. 1973. Optimal freezing conditions for cerebral metabolites in rats. J. Neurochem. 21:1127–1138.
Tzigaret, C., McIntosh, T. K., Okiyama, K., Jenkins, W. L., and Prasad, M. R. 1993. Measurement of hippocampal levels of cellular second messengers following in situ freezing. J. Neurochem. 60:827–834.
Bligh, E. G. and Dyer, W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917.
Dhillon, H. S., Carman, H. M., Zhang, D., Scheff, S. W., and Prasad, M. R. 1999. Severity of experimental brain injury on lactate and free fatty acid accumulation and Evans blue extravasation in the rat cortex and hippocampus. J. Neurotrauma 16:455–469.
Katayama, Y., Becker, D. P., Tamura, T., and Hovda, D. A. 1990. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73:889–900.
McIntosh, T. K. 1993. Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: A review. J. Neurotrauma 10:215–261.
Frandsen, A. and Schousboe, A. 1993. Excitatory amino acid-mediated cytotoxicity and calcium homeostasis in cultured neurons. J. Neurochem. 60:1202–1211.
Bally, P. R., Cahill, G. F., and Leboeuf, B. 1960. Studies on rat adipose tissue in vitro: V. Effects of glucose and insulin on themetabolism of palmitate-I-C14. J. Biol. Chem. 235:333.
Shapiro, B., Chowers, I., and Rose, G. 1955. Assimilation of fatty acids by adipose tissue. Page 347in Frazer, A. C. Biochemical Problems of Lipids, Butterworth, London & Interscience, New York.
Brewer, G. J. and Wallimann, T. W. 2000. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 74:1968–1978.
Ferrante, R. J., Andreassen, O. A., Jenkins, B. G., Dedeoglu, A., Kuemmerle, S., Kubilus, J. K., Kaddurah-Daouk, R., Hersch, S. M., and Beal, M. F. 2000. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 20:4389–4397.
Hausmann, O. N., Fouad, K., Wallimann, T., and Schwab, M. E. 2002. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord 40:449–456.
Zhang, W., Narayanan, M., and Friedlander, R. M. 2003. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann. Neurol. 53:267–270.
Ipsiroglu, O. S., Stromberger, C., Ilas, J., Hoger, H., Muhl, A., and Stockler-Ipsiroglu, S. 2001. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 69:1805–1815.
Guerrero-Ontiveros, M. L. and Wallimann, T. 1998. Creatine supplementation in health and disease: Effects of chronic creatine ingestion in vivo—Down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol. Cell Biochem. 184:427–437.
Brustovetsky, N., Brustovetsky, T., and Dubinsky, J. M. 2001. On the mechanisms of neuroprotection by creatine and phosphocreatine. J. Neurochem. 76:425–434.
Wallimann, T., Wyss, M., Brdiczka, D., Nicolay, K., and Eppenberger, H. M. 1992. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 281:21–40.
Rabow, L., DeSalles, A. F., Becker, D. P., Yang, M., Kontos, H. A., Ward, J. D., Moulton, R. J., Clifton, G., Gruemer, H. D., Muizelaar, J. P., et al. 1986. CSF brain creatine kinase levels and lactic acidosis in severe head injury. J. Neurosurg. 65:625–629.
Marmarou, A. 1992. Intracellular acidosis in human and experimental brain injury. J. Neurotrauma 9 (Suppl 2):S551–S562.
McIntosh, T. K., Faden, A. I., Bendall, M. R., and Vink, R. 1987. Traumatic brain injury in the rat: Alterations in brain lactate and pH as characterized by 1H and 31P nuclear magnetic resonance. J. Neurochem. 49:1530–1540.
Vink, R., McIntosh, T. K., Weiner, M. W., and Faden, A. I. 1987. Effects of traumatic brain injury on cerebral high-energy phosphates and pH: A 31P magnetic resonance spectroscopy study. J. Cereb. Blood Flow Metab. 7:563–571.
McIntosh, T. K., Vink, R., Noble, L., Yamakami, I., Fernyak, S., Soares, H., and Faden, A. L. 1989. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28:233–244.
Ishige, N., Pitts, L. H., Berry, I., Nishimura, M. C., and James, T. L. 1988. The effects of hypovolemic hypotension on high-energy phosphate metaboism of traumatized brain in rats. J. Neurosurg. 68:129–136.
Dhillon, H. S., Dose, J., and Prasad, M. R. 1998. Amphetamine administration improves neurochemical outcome of lateral fluid percussion injury in the rat. Brain Res. 804:231–237.
Prasad, M. R., Laabich, A., Dhillon, H. S., Zhang, L., Maki, A., Clerici, W. J., Hicks, R., Butcher, J., and Barron, S. 1997. Effects of six weeks of chronic ethanol administration on lactic acid accumulatiojn and high energy phosphate levels after experimental brain injury in rats. J. Neurotrauma 14:919–930.
Dhillon, H. S. and Prasad, M. R. 1999. Kynurenate attenuates the accumulation of diacylglycerol and free fatty acids after experimental brain injury in the rat. Brain Res. 832:7–12.
Gennarelli, T. A. 1994. Animate models of human head injury. J. Neurotrauma 11:357–368.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheff, S.W., Dhillon, H.S. Creatine-Enhanced Diet Alters Levels of Lactate and Free Fatty Acids After Experimental Brain Injury. Neurochem Res 29, 469–479 (2004). https://doi.org/10.1023/B:NERE.0000013753.22615.59
Issue Date:
DOI: https://doi.org/10.1023/B:NERE.0000013753.22615.59