Abstract
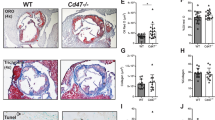
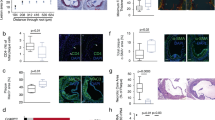
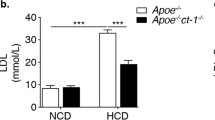
Atherosclerosis is a systemic disease of the large arteries, and activation of inflammatory pathways is important in its pathogenesis1. Increasing evidence supports the importance of CD40–CD154 interactions in atherosclerosis2,3, interactions originally known to be essential in major immune reactions4 and autoimmune diseases5. CD40 is present on atheroma-derived cells in vitro and in human atheromata in situ6. Ligation of CD40 on atheroma-associated cells in vitro activates the production of chemokines6, cytokines6, matrix metalloproteinases7,8, adhesion molecules9,10 and tissue factor7, substances responsible for lesion progression and plaque destabilization1. Administration of antibody against CD154 to low-density lipoprotein receptor-deficient mice has been shown to reduce atherosclerosis and decrease T-lymphocyte and macrophage content; however, only initial lesions were studied3. Here, we determined the effect of genetic disruption of CD154 in ApoE–/– mice in both initial and advanced atherosclerotic lesions. Plaque area was reduced 550%. In contrast to previous reports, initial lesion development was not affected. Advanced plaques in CD154–/–ApoE–/– mice had a less-lipid-containing, collagen-rich, stable plaque phenotype, with a reduced T-lymphocyte/macrophage content. These data indicate that CD40–CD154 signaling is important in late atherosclerotic changes, such as lipid core formation and plaque destabilization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ross, R. Atherosclerosis. An inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Mach, F., Schönbeck, U. & Libby, P. CD40 signaling in vascular cells: A key role in atherosclerosis? Atherosclerosis 137, S89–S95 (1998).
Mach, F., Schönbeck, U., Sukhova, G.K., Atkinson, E. & Libby, P. Reduction of atherosclerosis in mice by inhibition of CD40 signaling. Nature 394, 200–203 (1998).
Grewal, I.S. & Flavell, R.A. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol. Today 17, 410–414 (1996).
Aruffo, A. et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72, 291–300 (1993).
Mach, F. et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40L signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 94, 1931–1936 (1997).
Mach, F., Schönbeck, U., Bonnefoy, J.Y., Pober, J.S. & Libby, P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40. Induction of collagenase, stromelysin, and tissue factor. Circulation 96, 396–399 (1997).
Schönbeck, U. et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T-lymphocytes. A role for CD40 signaling in plaque rupture? Circ. Res. 81, 448–454 (1997).
Kornbluth, R., Kee, K. & Richman, D.D. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive β-chemokines. Proc. Natl. Acad. Sci. USA 95, 5205–5210 (1998).
Yellin, M.J. et al. Functional interactions of T cells with endothelial cells: The role of CD40L-CD40-mediated signals. J. Exp. Med. 182, 1857–1864 (1995).
Nakashima,Y, Plump, A.S., Raines, E.W., Breslow, J.L. & Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb, Vasc. Biol. 14, 133–140 (1994).
Stary, H.C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15, 1512–1531 (1995).
Lutgens, E. et al. Atherosclerosis in APOE*3 Leiden transgenic mice: from proliferative to atheromatous stage. Circulation 99, 276–283 (1999).
Johnson, R.C. et al. Absence of P-selectin delays fatty streak formation in mice. J. Clin. Invest. 99, 1037–1043 (1997).
Grewal, I.S., Xu, J. & Flavell, R.A.: Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature 378, 617–620 (1995).
Gupta, S. et al. IFN-τ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997).
Dansky, H.M., Charlton, S.A., Harper, M. & Smith, J.D. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA 94, 4642–4646 (1997).
Terkeltaub, R., Boisvert, W.A. & Curtiss, L.K. Chemokines and atherosclerosis. Curr. Opin. Lipidol. 9, 397–405 (1998).
Lutgens, E. et al. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res. 41, 473–479 (1999).
Dijkstra, C.D., Dopp, E.A., Joling, P. & Kraal,G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54, 589–599 (1985).
Acknowledgements
We thank C. Hughes for the original generation of the CD154 knockout mice. Part of this research was sponsored by the Wynand Pon Foundation, Leusden, the Netherlands. R.A.F. is an investigator and L.G. is an Associate of the Howard Hughes Medical Insitute. I.S.G. was supported by a Juvenile Diabetes Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lutgens, E., Gorelik, L., Daemen, M. et al. Requirement for CD154 in the progression of atherosclerosis. Nat Med 5, 1313–1316 (1999). https://doi.org/10.1038/15271
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15271
This article is cited by
-
Targeting inflammation in atherosclerosis — from experimental insights to the clinic
Nature Reviews Drug Discovery (2021)
-
The CD40-CD40L Dyad as Immunotherapeutic Target in Cardiovascular Disease
Journal of Cardiovascular Translational Research (2021)
-
Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease
Nature Communications (2021)
-
T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword
Nature Reviews Cardiology (2019)
-
Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates
Nature Biomedical Engineering (2018)