Abstract
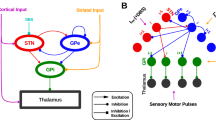
The subthalamic nucleus of the basal ganglia (STN) is important for normal movement1,2 as well as in movement disorders3,4,5. Lesioning6 or deep-brain stimulation7,8 of the STN can alleviate resting tremor in Parkinson's disease. The STN5 and its target nuclei9,10 display synchronized oscillatory burst discharge at low frequencies, some of which correlate with tremor, but the mechanism underlying this synchronized bursting is unknown. Here we show that the excitatory STN and inhibitory, external globus pallidus (GPe) form a feedback system that engages in synchronized bursting. In mature organotypic cortex–striatum–STN–GPe cultures, neurons in the STN and GPe spontaneously produce synchronized oscillating bursts at 0.4, 0.8 and 1.8 Hz. Pallidal lesion abolishes this bursting, whereas cortical lesion favours bursting at 0.8 Hz. Pallidal bursts, although weaker than STN bursts, were required for synchronized oscillatory burst generation by recruitment of subthalmic rebound excitation. We propose that the STN and GPe constitute a central pacemaker modulated by striatal inhibition of GPe neurons. This pacemaker could be responsible for synchronized oscillatory activity in the normal and pathological basal ganglia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Matsumura, M. Kojima, J., Gardiner, T. W. & Hikosaka, O. Visual and oculomotor functions of the monkey subthalamic nucleus. J. Neurophysiol. 67, 1615–1632 (1992).
Wichmann, T., Bergman, H. & DeLong, M. R. The primate subthalamic nucleus. I. Functional properties in intact animals. J. Neurophysiol. 72, 494–506 (1994).
Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Wichmann, T. & DeLong, M. R. Functional and pathophysiological models of the basal ganglia. Curr. Opin. Neurobiol. 6, 751–758 (1989).
Bergman, H., Wichmann, T., Karmon, B. & DeLong, M. R. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 72, 507–520 (1994).
Bergman, H., Wichmann, T. & DeLong, M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438 (1990).
Limousin, P.et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N. Engl. J. Med. 339, 1105–1111 (1998).
Rodriguez, M. C.et al. The subthalamic nucleus and tremor in Parkinson's disease. Mov. Disord. 13, 111–118 (1998).
Nini, A., Feingold, A., Slovin, H. & Bergman, H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of Parkinson. J. Neurophysiol. 74, 1800–1805 (1995).
Hurtado, J. M., Gray, C. M., Tamas, L. B. & Sigvardt, K. A. Dynamics of tremor-related oscillations in the human globus pallidus: A single case study. Proc. Natl Acad. Sci. USA 96, 1674–1679 (1999).
Plenz, D., Herrera-Marschitz, M. & Kitai, S. T. Morphological organization of the globus pallidus–subthalamic nucleus system studied in organotypic cultures. J. Comp. Neurol. 397, 437–457 (1998).
Kitai, S. T. & Deniau, J. M. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 214, 411–415 (1981).
Kita, H. Responses of globus pallidus neurons to cortical stimulation: intracellular study in the rat. Brain Res. 589, 84–90 (1992).
Kita, H., Chang, H. T. & Kitai, S. T. Pallidal inputs to subthalamus: intracellular analysis. Brain Res. 264, 255–265 (1983).
Parent, A. & Hazrati, L. N. Functional anatomy of the basal ganglia. II. The place of the subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Rev. 20, 128–154 (1995).
Shink, E., Bevan, M. D., Bolam, J. P. & Smith, Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience 73, 335–357 (1996).
Nakanishi, H., Kita, H. & Kitai, S. T. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain. Res. 437, 35–44 (1987).
Beurrier, C., Congar, P., Bioulac, B. & Hammond, C. Subthalamic nucleus neurons switch from single-spike activity to burst firing mode. J. Neurosci. 15, 599–609 (1999).
Ruskin, D. N.et al. Multisecond oscillations in firing rate in the basal ganglia: Robust modulation by dopamine receptor activation and anaesthesia. J. Neurophysiol. 81, 2046–2055 (1999).
DeLong, M. R. Activity of pallidal neurons during movement. J. Neurophysiol. 34, 414–427 (1971).
Filion, M. Effects of the interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res. 178, 425–441 (1978).
Aldridge, J. W. & Gilman, S. The temporal structure of spike trains in the primate basal ganglia: Afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res. 543, 123–138 (1991).
Hollerman, J. R. & Grace, A. A. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and resonse to haloperidol. Brain Res. 590, 291–299 (1992).
Hassani, O.-K., Mouroux, M. & Féger, J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neurosci. 72, 105–115 (1996).
Chesselet, M. F. & Delfs, J. M. Basal ganglia and movement disorders: an update. Trends Neurosci. 10, 417–422 (1996).
Pan, H. S. & Walters, J. R. Unilateral lesion of the nigrostriatal pathway decreases the firing rate and alters the firing pattern of globus pallidus neurons in the rat. Synapse 2, 650–656 (1988).
Miller, W. C. & DeLong, M. R. in The Basal Ganglia II (eds Carpenter, M. B. & Jayaraman, A.) 415–427 (Plenum, New York, 1987).
Wurtz, R. H. & Hikosaka, O. Role of the basal ganglia in the initiation of saccadic eye movements. Prog. Brain Res. 64, 175–190 (1986).
Chevalier, G. & Deniau, J. M. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 13, 277–280 (1990).
Abeles, M. Quantification, smoothing, and confidence limits for single-units' histograms. J. Neurosci. Methods 5, 317–325 (1982).
Acknowledgements
We thank B. Teng for technical assistance with the preparation of cultures and for immunohistochemistry, and H. Steiner for discussions. This work was supported by grants from the National Parkinson Foundation (to D.P.) and the NINDS (to D.P. and S.T.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plenz, D., Kital, S. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400, 677–682 (1999). https://doi.org/10.1038/23281
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/23281
This article is cited by
-
Dynamical mechanism of parkinsonian beta oscillation in a heterogenous subthalamopallidal network
Nonlinear Dynamics (2023)
-
A model description of beta oscillations in the external globus pallidus
Cognitive Neurodynamics (2023)
-
Angiotensin II increases the firing activity of pallidal neurons and participates in motor control in rats
Metabolic Brain Disease (2023)
-
Dynamics of phase oscillator networks with synaptic weight and structural plasticity
Scientific Reports (2022)
-
Subthalamic nucleus stabilizes movements by reducing neural spike variability in monkey basal ganglia
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.