Key Points
-
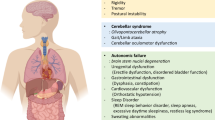
Parkinson's disease (PD) is the second most common neurodegenerative disease. It is characterized by degeneration of dopamine neurons in the substantia nigra and by the presence of intracytoplasmic inclusions known as Lewy bodies, which contain a protein known as α-synuclein. Several animal models of this neurodegenerative disorder have been developed. Each of them has strengths and limitations, which are the focus of this review.
-
An ideal model of PD should have the following characteristics: 1) A normal complement of dopamine neurons at birth with selective and gradual loss of commencing in adulthood. 2) Easily detectable motor deficits, including bradykinesia, rigidity and resting tremor. 3) It should show the development of Lewy bodies. 4) If the model is genetic, it should be based on a single mutation to allow for the robust propagation of the mutation. 5) It should have a relatively short disease course of a few months, allowing rapid screening of therapeutic agents.
-
Injection of 6-hydroxydopamine into the substantia nigra is an early model of PD. This drug kills dopamine neurons owing to the generation of free radicals. This model has been useful for pharmacological screening but it is not characterized by the gradual loss of neurons nor by the formation of Lewy bodies. However, a significant advantage of this model is the presence of a quantifiable motor deficit — rotation.
-
A model based on the injection of rotenone has also been developed. This toxin likely kills neurons by oxidative damage. The rotenone model is characterized by a progressive degeneration of nigrostriatal neurons, by the presence cytoplasmic inclusions reminiscent of Lewy bodies and by the appearance of motor deficits. It suffers, however, from a large variability in susceptibility of individual animals to the toxin, a variability that precludes usefulness of the model to test neuroprotective agents.
-
A novel model of PD was produced by expressing α-synuclein in Drosophila melanogaster. A subset of dopamine neurons disappeared in the mutant flies. Inclusions that resembled Lewy bodies were also observed, and the flies developed locomotor dysfunction with age, although it is not clear whether the motor deficits are due to dopamine dysfunction. In addition, no differences in toxicity were observed between wild-type and mutant α-synuclein expression. But the main advantage of this model is the well-characterized genetics of Drosophila, which will allow for the characterization of enhancer and suppressor mutations.
-
Transgenic mice overexpressing α-synuclein under the control of a Thy-1 promoter show progressive loss of motor function and the accumulation of Lewy-body-like inclusions although not in substantia nigra. This model therefore produces some aspects of the pathology of dementia with Lewy bodies, but it does not faithfully replicate PD.
-
A model based on MPTP toxicity in primates replicates all the clinical signs of PD, including tremor, rigidity, akinesia and postural instability. The substantia nigra is particularly vulnerable to the generation of free radicals that is caused by the drug. The main difficulty with MPTP, however, is that it is an acute or subacute process. In addition, Lewy-body-formation is also absent.
-
Available models of PD have contributed greatly to our understanding of the pathophysiology and potential therapeutics for this condition but, on the basis of the five basic considerations put forward as the hallmarks of an ideal model, it is clear that this model is not available yet. Advances in genetics will probably lead to improved models of PD over the next few years. This will contribute to an improved understanding of the pathophysiology of this condition, as well as to the development of novel therapeutic strategies.
Abstract
Research into the pathogenesis of Parkinson's disease has been rapidly advanced by the development of animal models. Initial models were developed by using toxins that specifically targeted dopamine neurons, the most successful of which used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a toxin that causes parkinsonism in man. More recently, the identification of α-synuclein mutations as a rare cause of Parkinson's disease has led to the development of α-synuclein transgenic mice and Drosophila. Here, I discuss the merits and limitations of these different animal models in our attempts to understand the physiology of Parkinson's disease and to develop new therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lang, A. E. & Lozano, A. M. Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044– 1053 (1998).
Lang, A. E. & Lozano, A. M. Parkinson's disease. Second of two parts. N. Engl. J. Med. 339, 1130– 1143 (1998).
Gorell, J. M., Johnson, C. C., Rybicki, B. A., Peterson, E. L. & Richardson, R. J. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology 50, 1346– 1350 (1998).
Seidler, A. et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology 46, 1275–1284 (1996).
Sveinbjornsdottir, S. et al. Familial aggregation of Parkinson's disease in Iceland. N. Engl. J. Med. 343, 1765–1770 (2000).Provides strong evidence for genetic factors that might have partial penetrance in the pathogenesis of Parkinson's disease.
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045 –2047 (1997).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605– 608 (1998).
Ungerstedt, U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol. Scand. 367, S49–S68 (1971).
Sauer, H. & Oertel, W. H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59, 401–415 (1994).
Menegon, A., Board, P. G., Blackburn, A. C., Mellick, G. D. & Le Couteur, D. G. Parkinson's disease, pesticides, and glutathione transferase polymorphisms. Lancet 352 , 1344–1346 (1998).
Caparros-Lefebvre, D. & Elbaz, A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case-control study. Caribbean Parkinsonism Study Group. Lancet 354, 281–286 ( 1999).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 3, 1301–1306 (2000). Describes a novel model of Parkinson's disease using intravenous infusion of the insecticide rotenone in rats. This compound is a selective complex I inhibitor of an electron transport chain, yet the damage is confined to the substantia nigra.
Ouary, S. et al. Major strain differences in response to chronic systemic administration of the mitochondrial toxin 3-nitropropionic acid in rats: implications for neuroprotection studies. Neuroscience 97, 521–530 (2000).
Beal, M. F. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 23, 294–300 (2000).
Feany, M. B. & Bender, W. W. A Drosophila model of Parkinson's disease. Nature 404, 394– 298 (2000).The first Drosophila model of overexpression of both mutant and wild-type α-synuclein. Interestingly, there was a selective loss of dopamine neurons and neuronal inclusions that resembled Lewy bodies.
Abeliovich, A. et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000).
Masliah, E. et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 (2000).
van der Putten, H. et al. Neuropathology in mice expressing human α-synuclein . J. Neurosci. 20, 6021– 6029 (2000).
Bloem, B. R. et al. The MPTP model: versatile contributions to the treatment of idiopathic Parkinson's disease. J. Neurol. Sci. 97, 273–293 (1990).
Bezard, E. et al. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp. Neurol. 155, 268–273 (1999).
Takahashi, N. et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl Acad. Sci. USA 94, 9938– 9943 (1997).
Tipton, K. F. & Singer, T. P. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 61, 1191–1206 (1993).
Chan, P., DeLanney, L. E., Irwin, I., Langston, J. W. & DiMonte, D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J. Neurochem. 57, 348– 351 (1991).
Klivenyi, P. et al. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol. Dis. 5, 253– 258 (1998).
Przedborski, S. et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 12, 1658– 1667 (1992).
Andreassen, O. A. et al. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 167, 189– 195 (2001).
Klivenyi, P. et al. Inhibition of neuronal nitric oxide synthase protects against MPTP toxicity. Neuroreport 11, 1265– 1268 (2000).
Zhang, J., Graham, D. G., Montine, T. J. & Ho, Y. S. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J. Neuropathol. Exp. Neurol. 59, 53– 61 (2000).
Pennathur, S., Jackson-Lewis, V., Przedborski, S. & Heinecke, J. W. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J. Biol. Chem. 274, 34621–34628 ( 1999).
Schulz, J. B., Matthews, R. T., Muqit, M. M. K., Browne, S. E. & Beal, M. F. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J. Neurochem. 64, 936– 939 (1995).
Good, P. F., Hsu, A., Werner, P., Perl, D. P. & Olanow, C. W. Protein nitration in Parkinson's disease. J. Neuropathol. Exp. Neurol. 57, 338– 339 (1998).
Hantraye, P. et al. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nature Med. 2, 1017–1021 (1996). The first paper to show that an inhibitor of neuronal nitric oxide synthase produces marked neuroprotective effects against the clinical biochemical and neuropathological effects of MPTP in primates.
Matthews, R. T., Yang, L. & Beal, M. F. S-methylthiocitrulline, a neuronal nitric oxide synthase inhibitor, protects against malonate and MPTP neurotoxicity. Exp. Neurol. 143, 282–286 (1997).
Przedborski, S. et al. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc. Natl Acad. Sci. USA 93, 4565–4571 ( 1996).Showed that mice deficient in neuronal nitric oxide synthase were partially resistant to MPTP neurotoxicity, consistent with a role of peroxynitrite.
Dehmer, T., Lindenau, J., Haid, S., Dichgans, J. & Schulz, J. B. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J. Neurochem. 74, 2213–2216 (2000).
Liberatore, G. T. et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nature Med. 5, 1403–1409 (1999). Showed that in mice deficient in inducible nitric oxide synthase, dopamine cell bodies were protected from MPTP toxicity but the dopamine terminals were not spared.
Cosi, C. & Marien, M. Decreases in mouse brain NAD+ and ATP induced by 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP): prevention by the poly(ADP-ribose) polymerase inhibitor, benzamide . Brain Res. 809, 58–67 (1998).
Mandir, A. S. et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc. Natl Acad. Sci. USA 96, 5774–5779 (1999).Mice deficient in poly(ADP-ribose) polymerase are resistant to MPTP, implicating it in cell death. This enzyme is activated by oxidative damage to DNA.
Schulz, J. B., Henshaw, D. R., Matthews, R. T. & Beal, M. F. Coenzyme Q10 and nicotinamide and a free radical spin trap protect against MPTP neurotoxicity. Exp. Neurol. 132, 279–283 (1995).
Burns, R. S. et al. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N -methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl Acad. Sci. USA 80, 4546–4550 ( 1983).
Varastet, M., Riche, D., Maziere, M. & Hantraye, P. Chronic MPTP treatment reproduces in baboons the differential vulnerability of mesencephalic dopaminergic neurons observed in Parkinson's disease. Neuroscience 63, 47–56 ( 1994).
Forno, L. S., Langston, J. W., DeLanney, L. E., Irwin, I. & Ricaurte, G. A. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann. Neurol. 20 , 449–455 (1986).
Forno, L. S., Langston, J. W., DeLanney, L. E. & Irwin, I. An electron microscopic study of MPTP-induced inclusion bodies in an old monkey . Brain Res. 448, 150–157 (1988).
Albanese, A. et al. Chronic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to monkeys: behavioural, morphological and biochemical correlates. Neuroscience 55, 823–832 (1993).
Brownell, A. L. et al. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nature Med. 4, 1308–1312 (1998).
Hantraye, P. et al. Stable parkinsonian syndrome and uneven loss of striatal dopamine fibers following chronic MPTP administration in baboons. Neuroscience 53, 169–178 ( 1993).
Bankiewicz, K. S. et al. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci. 39, 7–16 ( 1986).
Bergman, H., Wichmann, T. & DeLong, M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438 (1990).
Wichmann, T., Kliem, M. A. & DeLong, M. R. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp. Neurol. 167, 410–424 (2001).
Tatton, N. A. & Kish, S. J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77, 1037– 1048 (1997).
Hartmann, A. et al. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc. Natl Acad. Sci. USA 97, 2875–2880 (2000).
Tatton, N. A. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp. Neurol. 166, 29–43 (2000).
Wullner, U. et al. Cell death and apoptosis regulating proteins in Parkinson's disease — a cautionary note. Acta Neuropathol. (Berl.) 97, 408–412 (1999).
Yang, L. et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J. Neurosci. 18, 8145–8152 (1998). First paper to show that an anti-apoptotic protein can protect against both acute and chronic MPTP toxicity.
Offen, D. et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity . Proc. Natl Acad. Sci. USA 95, 5789– 5794. (1998).
Klivenyi, P. et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Med. 5, 347–350 (1999).
Vila, M. et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 98, 2837– 2842 (2001).
Eberhardt, O. et al. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Neurosci. 20, 9126– 9134 (2000).Shows that an inhibitor of apoptosis preferentially protects dopamine cell bodies, whereas the growth factor GDNF protects dopamine terminals.
Trimmer, P. A., Smith, T. S., Jung, A. B. & Bennett, J. P. Jr, Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5, 233–239 (1996).
Saporito, M. S., Brown, E. M., Miller, M. S. & Carswell, S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J. Pharmacol. Exp. Ther. 288 , 421–427 (1999).
Saporito, M. S., Thomas, B. A. & Scott, R. W. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo . J. Neurochem. 75, 1200– 1208 (2000).
Waldmeier, P. C., Spooren, W. P. & Hengerer, B. CGP 3466 protects dopaminergic neurons in lesion models of Parkinson's disease. Naunyn Schmiedebergs Arch. Pharmacol. 362, 526–537 (2000).
Langston, J. W. et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure . Ann. Neurol. 46, 598– 605 (1999).
Kurkowska-Jastrzebska, I., Wronska, A., Kohutnicka, M., Czlonkowski, A. & Czlonkowska, A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 156, 50–61 (1999).
Teismann, P. & Ferger, B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson's disease. Synapse 39, 167– 174 (2001).
Klivenyi, P. et al. Mice deficient in group IV cytosolic phospholipase A2 are resistant to MPTP neurotoxicity. J. Neurochem. 71, 2634–2637 (1998).
Ferger, B., Teismann, P., Earl, C. D., Kuschinsky, K. & Oertel, W. H. Salicylate protects against MPTP-induced impairments in dopaminergic neurotransmission at the striatal and nigral level in mice. Naunyn Schmiedebergs Arch. Pharmacol. 360, 256–261 (1999).
Mohanakumar, K. P., Muralikrishnan, D. & Thomas, B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Brain Res. 864, 281– 290 (2000).
Brouillet, E. & Beal, M. F. NMDA antagonists partially protect against MPTP induced neurotoxicity in mice. Neuroreport 4, 387–390 (1993).
Lange, K. W. et al. The competitive NMDA antagonist CPP protects substantia nigra neurons from MPTP-induced degeneration in primates. Naunyn Schmiedebergs Arch. Pharmacol. 348, 586–592 (1993).
Zuddas, A. et al. MK-801 prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinine-induced parkinsonism in primates. J. Neurochem. 59, 733–739 (1992).
Lau, Y. S. & Mouradian, M. M. Protection against acute MPTP-induced dopamine depletion in mice by adenosine A1 agonist. J. Neurochem. 60, 768–771 ( 1993).
Benazzouz, A. et al. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study. Eur. J. Pharmacol. 284, 299–307 (1995).
Boireau, A. et al. Riluzole and experimental parkinsonism: antagonism of MPTP-induced decrease in central dopamine levels in mice. Neuroreport 5, 2657–2660 (1994).
Muralikrishnan, D. & Mohanakumar, K. P. Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J. 12, 905– 912 (1998).
Zou, L. et al. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci. Lett. 281, 167 –170 (2000).
Moussaoui, S. et al. The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson's disease. Exp. Neurol. 166, 235–245 ( 2000).
Matthews, R. T. et al. Novel free radical spin traps protect against malonate and MPTP neurotoxicity. Exp. Neurol. 157, 120 –126 (1999).
Callier, S., Morissette, M., Grandbois, M. & Di Paolo, T. Stereospecific prevention by 17β-estradiol of MPTP-induced dopamine depletion in mice. Synapse 37, 245– 251 (2000).
Genc, S. et al. Erythropoietin exerts neuroprotection in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/BL mice via increasing nitric oxide production. Neurosci. Lett. 298, 139–141 ( 2001).
Costa, S., Iravani, M. M., Pearce, R. K. & Jenner, P. Glial cell line-derived neurotrophic factor concentration dependently improves disability and motor activity in MPTP-treated common marmosets. Eur. J. Pharmacol. 412, 45–50 (2001).
Gash, D. M. et al. Functional recovery in parkinsonian monkeys treated with GDNF . Nature 380, 252–255 (1996).
Tomac, A. et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335– 339 (1995).First paper to show that GDNF has restorative effects on dopamine neurons after MPTP treatment.
Kordower, J. H. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 (2000). Showed that administration of GDNF using a lentiviral vector in primates was highly effective against MPTP toxicity.
Costantini, L. C. et al. A novel immunophilin ligand: distinct branching effects on dopaminergic neurons in culture and neurotrophic actions after oral administration in an animal model of Parkinson's disease. Neurobiol. Dis. 5, 97–106 (1998).
Steiner, J. P. et al. Neurotrophic immunophilin ligands stimulate structural and functional recovery in neurodegenerative animal models. Proc. Natl Acad. Sci. USA 94, 2019–2024 (1997).References 85 and 86 show that immunophilins produce restorative effects after MPTP treatment.
Bodis-Wollner, I. et al. Acetyl-levo-carnitine protects against MPTP-induced parkinsonism in primates. J. Neural. Transm. Park. Dis. Dement. Sect. 3, 63–72 (1991).
Beal, M. F., Matthews, R., Tieleman, A. & Schults, C. W. Coenzyme Q10 attenuates the MPTP induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 783 , 109–114 (1997).
Shults, C. W., Haas, R. H., Passov, D. & Beal, M. F. Coenzyme Q 10 is reduced in mitochondria from parkinsonian patients. Ann. Neurol. 42, 261–264 (1997).
Kowall, N. W. et al. MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. Neuroreport 11, 211– 213 (2000).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice . Cell 87, 493–506 (1996).
Hashimoto, M. et al. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport 10, 717–721 (1999).
Shimura, H. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature Genet. 25, 302– 305 (2000).
Vila, M. et al. α-synuclein upregulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J. Neurochem. 74, 721–729 (2000).
Przedborski, S. et al. Oxidative post-translational modifications of α-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J. Neurochem. 76, 637 –640 (2001).
Giasson, B. I. et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000). Used antibodies to nitrated α-synuclein to show that the α-synuclein in Lewy bodies in Parkinson's disease is nitrated, consistent with oxidative damage mediated by peroxynitrite.
Hsu, L. J. et al. α-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157, 401– 410 (2000).
Lee, M., Hyun, D. H., Halliwell, B. & Jenner, P. Effect of the overexpression of wild-type or mutant α-synuclein on cell susceptibility to insult. J. Neurochem. 76, 998–1009 (2001).
Bergman, H., Wichmann, T., Karmon, B. & DeLong, M. R. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism . J. Neurophysiol. 72, 507– 520 (1994).
Filion, M., Tremblay, L. & Bedard, P. J. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 547, 152–161 (1991).
Wichmann, T. et al. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp. Brain Res. 125, 397–409 (1999).
Bronstein, J. M., DeSalles, A. & DeLong, M. R. Stereotactic pallidotomy in the treatment of Parkinson disease: an expert opinion. Arch. Neurol. 56, 1064–1069 (1999).
Limousin, P. et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N. Engl. J. Med. 339, 1105–1111 (1998).
Forno, L. S. Neuropathology of Parkinson's disease. J. Neuropathol. Exp. Neurol. 55, 259–272 ( 1996).
Acknowledgements
The secretarial assistance of Sharon Melanson is gratefully acknowledged. This work was supported by the National Institutes of Health, the Department of Defense, The Parkinson's Disease Foundation, The ALS Association, The Hereditary Disease Foundation and The Huntington's Disease Society.
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- BRADYKINESIA
-
Slowing of and difficulty in initiating movement that is characteristic of Parkinson's disease.
- AMPHETAMINE
-
Molecule that inhibits dopamine uptake and increases the level of this transmitter in the cleft. Its intake by normal subjects induces a psychotic state.
- POLYMORPHISM
-
The simultaneous existence in the same population of two or more genotypes in frequencies that cannot be explained by recurrent mutations.
- ROTAROD TEST
-
Motor test that probes the ability of rodents to keep their balance on a cylinder that rotates continuously at a slow speed, commonly 5–6 revolutions per minute.
- LENTIVIRUSES
-
A group of retroviruses that include HIV. Virus derivatives that are engineered to be replication-defective can be used as expression vectors. Lentiviral vectors have advantages over retroviral vectors because of their ability to infect non-dividing human cells, particularly neurons.
Rights and permissions
About this article
Cite this article
Beal, M. Experimental models of Parkinson's disease . Nat Rev Neurosci 2, 325–332 (2001). https://doi.org/10.1038/35072550
Issue Date:
DOI: https://doi.org/10.1038/35072550
This article is cited by
-
Mitochondrial complex I inhibition triggers NAD+-independent glucose oxidation via successive NADPH formation, “futile” fatty acid cycling, and FADH2 oxidation
GeroScience (2024)
-
Neuroprotective effects of osmotin in Parkinson’s disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways
Journal of Biomedical Science (2023)
-
Prediction analysis for Parkinson disease using multiple feature selection & classification methods
Multimedia Tools and Applications (2023)
-
Brain regions susceptible to alpha-synuclein spreading
Molecular Psychiatry (2022)
-
Examination of diverse iron-chelating agents for the protection of differentiated PC12 cells against oxidative injury induced by 6-hydroxydopamine and dopamine
Scientific Reports (2022)