Abstract
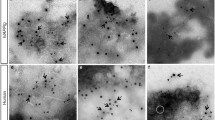
THE 37-amino-acid polypeptide amylin is the principal constituent of the amyloid deposits that form in the islets of Langerhans in patients with type-2 diabetes mellitus1–5, but its role in the pathogenesis of this disease is unresolved6–8. In view of the fact that the β-amyloid protein that forms fibrils in Alzheimer's disease is toxic to neurons 9,10, we have investigated whether amylin fibrils could be toxic to pancreatic islet cells. We show here that human amylin is toxic to insulin-producing β-cells of the adult pancreas of rats and humans. This toxicity is mediated by the fibrillar form of the amylin peptide and requires direct contact of the fibrils with the cell surface. The mechanism of cell death involves RNA and protein synthesis and is characterized by plasma membrane blebbing, chromatin condensation and DNA fragmentation, indicating that amylin induces islet cell apoptosis. These findings indicate that amylin fibril formation in the pancreas may cause islet cell dysfunction and death in type-2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Opie, E. L. J. exp. Med. 5, 397–428 (1900).
Cooper, G. J. S. et al. Proc. natn. Acad. Sci. U.S.A. 84, 8628–8632 (1987).
Westermark, P. et al. Proc. natn. Acad. Sci. U.S.A. 84, 3881–3885 (1987).
Westermark, P., Wernstedt, C., O'Brien, T. D., Hayden, D. W. & Johnson, K. H. Am. J. Path. 127, 414–417 (1987).
Cooper, G. J. S. et al. Proc. natn. Acad. Sci. U.S.A. 85, 7763–7766 (1988).
Leighton, B. & Cooper, G. J. S. Nature 335, 632–635 (1988).
Johnson, K. H., O'Brien, T. D., Betsholtz, C. & Westermark, P. New Engl. J. Med. 321, 513–518 (1989).
O'Brien, T. D., Butler, P. C., Westermark, P. & Johnson, K. H. Vet. Path. 30, 317–332 (1993).
Yankner, B. A. et al. Science 245, 417–420 (1989).
Yankner, B. A., Duffy, L. K. & Kirschner, D. A. Science 250, 279–282 (1990).
May, P. C., Boggs, L. N. & Fuson, K. S. J. Neurochem. 61, 2330–2333 (1993).
Fraser, P. E., Nguyen, W. K., Surewicz, W. D. & Kirschner, D. A. Biophys. J. 60, 1190–1201 (1991).
Westermark, P., Engstrom, U., Johnson, K. H., Westermark, G. T. & Betsholtz, C. Proc. natn. Acad. Sci. U.S.A. 87, 5036–5040 (1990).
Betsholtz, C. et al. FEBS Lett. 251, 261–264 (1989).
Leffert, J. D., Newgard, C. B., Okamoto, H., Milburn, J. L. & Luskey, K. L. Proc. natn. Acad. Sci. U.S.A. 86, 3127–3130 (1989).
Nishi, M., Chan, S. J., Nagamatsu, S., Belle, G. I. & Steiner, D. F. Proc. natn. Acad. Sci. U.S.A. 86, 5738–5742 (1989).
Batistatou, A. & Greene, L. A. J. Cell Biol. 115, 461–471 (1991).
Wyllie, A. H., Kerr, F. F. R. & Currie, A. R. Int. Rev. Cytol. 68, 251–306 (1980).
Maloy, A. L., Longnecker, D. S. & Greenberg, E. R. Hum. Path. 12, 917–922 (1981).
Westermark, P. Uppsala J. Med. Sci. 77, 91–94 (1972).
Yankner, B. A. & Mesulam M.-M. New Engl. J. Med. 325, 1849–1857 (1991).
Forloni, G. et al. Nature 362, 543–546 (1993).
DeArmond, S. J. et al. Cell 41, 221–235 (1985).
Forloni, G. et al. NeuroReport 4, 523–526 (1993).
Takashima, A., Noguchi, K., Sato, K., Hoshino, T. & Imahori, K. Proc. natn. Acad. Sci. U.S.A. 90, 7789–7793 (1993).
Loo, D. T. et al. Proc. natn. Acad. Sci. U.S.A. 90, 7951–7955 (1993).
Schuppin, G. T., Bonner-Weir, S., Montana, E., Kaiser, N. & Weir G. C. In Vitro Cell dev. Biol. 29, 339–344 (1993).
Ricordi, D. et al. Surgery 107, 688–694 (1990).
Weston, S. A. & Parish, C. R. J. immunol. Meth. 133, 87–95 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lorenzo, A., Razzaboni, B., Weir, G. et al. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368, 756–760 (1994). https://doi.org/10.1038/368756a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/368756a0
This article is cited by
-
Mammalian models of diabetes mellitus, with a focus on type 2 diabetes mellitus
Nature Reviews Endocrinology (2023)
-
Exposure to Endocrine-Disrupting Chemicals and Type 2 Diabetes Mellitus in Later Life
Exposure and Health (2023)
-
Islet amyloid polypeptide aggregation exerts cytotoxic and proinflammatory effects on the islet vasculature in mice
Diabetologia (2022)
-
Optogenetic approaches for understanding homeostatic and degenerative processes in Drosophila
Cellular and Molecular Life Sciences (2021)
-
Revealing the Mechanism of EGCG, Genistein, Rutin, Quercetin, and Silibinin Against hIAPP Aggregation via Computational Simulations
Interdisciplinary Sciences: Computational Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.