Abstract
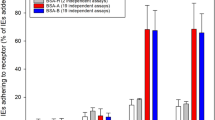
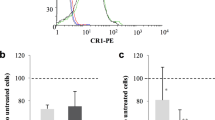
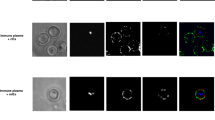
The factors determining disease severity in malaria are complex and include host polymorphisms, acquired immunity and parasite virulence1. Studies in Africa have shown that severe malaria is associated with the ability of erythrocytes infected with the parasite Plasmodium falciparum to bind uninfected erythrocytes and form rosettes2,3,4,5. The molecular basis of rosetting is not well understood, although a group of low-molecular-mass proteins called rosettins have been described as potential parasite ligands6. Infected erythrocytes also bind to endothelial cells, and this interaction is mediated by the parasite-derived variant erythrocyte membrane protein PfEMP1 (refs 7, 8), which is encoded by the var gene family9,10,11. Here we report that the parasite ligand for rosetting in a P. falciparum clone is PfEMP1, encoded by a specific var gene. We also report that complement-receptor 1 (CR1) on erythrocytes plays a role in the formation of rosettes and that erythrocytes with a common African CR1 polymorphism (Sl(a−))12 have reduced adhesion to the domain of PfEMP1 that binds normal erythrocytes. Thus we describe a new adhesive function for PfEMP1 and raise the possibility that CR1 polymorphisms in Africans that influence the interaction between erythrocytes and PfEMP1 may protect against severe malaria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Greenwood, B., Marsh, K. & Snow, R. Why do some African children develop severe malaria? Parasitol Today 7, 277–281 (1991).
Carlson, J. et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336, 1457–1460 (1990).
Treutiger, C. J. et al. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am. J. Trop. Med. Hyg. 46, 503–510 (1992).
Ringwald, P. et al. Parasite virulence factors during falciparum malaria rosetting, cytoadherence, and modulation of cytoadherence by cytokines. Infect. Immun. 61, 5198–5204 (1993).
Rowe, A., Obeiro, J., Newbold, C. I. & Marsh, K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 63, 2323–2326 (1995).
Helmby, H., Cavelier, L., Pettersson, U. & Wahlgren, M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect. Immun. 61, 284–288 (1993).
Baruch, D. I., Gromley, J. A., Ma, C., Howard, R. J. & Pasloske, B. L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl Acad. Sci. USA 93, 3497–3502 (1996).
Gardner, J. P., Pinches, R. A., Roberts, D. J. & Newbold, C. I. Variant antigens and endothelial receptor adhesium in Plasmodium falciparum. Proc. Natl Acad. Sci. USA 93, 3503–3508 (1996).
Baruch, D. I. et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Smith, J. D. et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected ethrocytes. Cell 82, 101–110 (1995).
Su, X.-Z. et al. Alarge and diverse family gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–99 (1995).
Moulds, M. K. Serological investigation and clinical significance of high-titer, low-avidity (HTLA) antibodies. Am. Soc. Med. Technol. 47, 789–794 (1981).
Handunnetti, S. M. et al. Purification and in vivo selection of rosette-positive (R+) and rosette-negative (R−) phenotypes of knob-positive. Plasmodium falciparum parasites. Am. J. Trop. Med. Hyg. 46, 371–381 (1992).
Roberts, D. J. et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357, 689–692 (1992).
Arnold, C. & Hodgson, I. J. Vectorette PCR: a novel approach to genome walking. PCR Meth. Appl. 1, 39–42 (1991).
Chitnis, C. E. & Miller, L. H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 180, 497–506 (1994).
Cohen, G. H. et al. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J. Virus. 62, 1932–1940 (1988).
Handunnetti, S. M. et al. Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood 80, 2097–2104 (1992).
Rowe, A., Berendt, A. R., Marsh, K. & Newbold, C. I. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocytes rosettes. Exp. Parasitol. 79, 506–516 (1994).
Moulds, J. M., Moulds, J. J., Brown, M. & Atkinson, J. P. Antiglobulin testing for CR1-related (Knops/McCoy/Swain-Langley/York) blood group antigens: negative and weak reactions are caused by variable expression of CR1. Vox Sanguinis 62, 230–235 (1992).
Moulds, J. M., Nickells, M. W., Moulds, J. J., Brown, M. C. & Atkinson, J. P. The C3b/C4b receptor is recognized by the Knops, McCoy, Swain-Langley and York blood group antisera. J. Exp. Med. 173, 1159–1163 (1991).
Carlson, J. & Wahlgren, M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J. Exp. Med. 176, 1311–1317 (1992).
Weisman, H. F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990).
Hill, A. V. S. Malaria resistance genes: a natural selection. Trans. R. Soc. Trop. Med. Hyg. 1992, 225–226 (1992).
Miller, L. H. Impact of malaria on genetic polymorphism and genetic diseases in Africans and African Americans. Proc. Natl Acad. Sci. USA 91, 2415–2419 (1994).
Scholander, C., Treutiger, C. J., Hultenby, K. & Wahlgren, M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nature Med. 2, 204–208 (1996).
Peterson, D. S., Miller, L. H. & Wellems, T. E. Isolation of multiple sequences from the Plasmodium falciparum genome that encode conserved domains homologous to those in erythrocyte-binding proteins. Proc. Natl Acad. Sci. USA 92, 7100–7104 (1995).
Yadava, A., Kumar, S., Dvorak, J. A., Milon, G. & Miller, L. H. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc. Natl Acad. Sci. USA 93 4595–4599 (1996).
Vengelen-Tyler, V. AABB Technical manual(American Association of Blood Banks, Bethesda, (1996)).
Acknowledgements
We thank J. Proctor, D. Mallory, L. McCall and B. Smith for negative and null erythrocytes; M. Wahlgren and R. Howard for parasite clones/lines; G. Cohen and R. Eisenberg for the pRE4 plasmid and monoclonal antibodies; J. Smith for the A4var expression construct; D. Fearon for sCR1; D. Alling for statistical advice; and B. Sullivan for assistance in identifying Sl(a−) donors. This work was funded by the Wellcome Trust (J.A.R. and C.I.N.), the National Blood Foundation (J.M.M.), and the Arthritis Foundation (J.M.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rowe, J., Moulds, J., Newbold, C. et al. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388, 292–295 (1997). https://doi.org/10.1038/40888
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40888
This article is cited by
-
Elucidation of the low-expressing erythroid CR1 phenotype by bioinformatic mining of the GATA1-driven blood-group regulome
Nature Communications (2023)
-
Diagnosis of malaria in pregnancy: accuracy of CareStart™ malaria Pf/PAN against light microscopy among symptomatic pregnant women at the Central Hospital in Yaoundé, Cameroon
Malaria Journal (2022)
-
Red blood cell blood group A antigen level affects the ability of heparin and PfEMP1 antibodies to disrupt Plasmodium falciparum rosettes
Malaria Journal (2021)
-
Insights on Rosetting Phenomenon in Plasmodium vivax Malaria
Current Clinical Microbiology Reports (2021)
-
Safety, infectivity and immunogenicity of a genetically attenuated blood-stage malaria vaccine
BMC Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.