Abstract
Acute myeloid leukemia (AML) is currently treated with aggressive chemotherapy that is not well tolerated in many elderly patients, hence the unmet medical need for effective therapies with less toxicity and better tolerability. Inhibitors of FMS-like tyrosine kinase 3 (FLT3), JAK2 and histone deacetylase inhibitors (HDACi) have been tested in clinical studies, but showed only moderate single-agent activity. High efficacy of the HDACi pracinostat treating AML and synergy with the JAK2/FLT3 inhibitor pacritinib is demonstrated. Both compounds inhibit JAK-signal transducer and activator of transcription (STAT) signaling in AML cells with JAK2V617F mutations, but also diminish FLT3 signaling, particularly in FLT3-ITD (internal tandem duplication) cell lines. In vitro, this combination led to decreased cell proliferation and increased apoptosis. The synergy translated in vivo in two different AML models, the SET-2 megakaryoblastic AML mouse model carrying a JAK2V617F mutation, and the MOLM-13 model of FLT3-ITD-driven AML. Pracinostat and pacritinib in combination showed synergy on tumor growth, reduction of metastases and synergistically decreased JAK2 or FLT signaling, depending on the cellular context. In addition, several plasma cytokines/growth factors/chemokines triggered by the tumor growth were normalized, providing a rationale for combination therapy with an HDACi and a JAK2/FLT3 inhibitor for the treatment of AML patients, particularly those with FLT3 or JAK2 mutations.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a myeloid malignancy characterized by deregulated proliferation, increased self-renewal and limited differentiation of myeloid blasts. AML is typically diagnosed in elderly patients and the standard of care treatment is mainly chemotherapy. Most patients relapse and perish from the disease or the associated complications. Aggressive chemotherapeutic treatment can only be used in a minority of patients; hence, there is a great medical unmet need, for effective targeted therapy with less toxicity and better tolerability.1
Histone deacetylase inhibitors (HDACi) are a class of drugs that alter the acetylation status of both histone and non-histone proteins, thereby affecting a range of cellular functions of neoplastic cells, such as transcriptional activation, cell proliferation, immune responses, cell differentiation, survival and angiogenesis.2, 3 HDACis, including pracinostat (SB939), have shown clinical activity in AML and myelodysplastic syndrome, as well as myeloproliferative neoplasms (MPNs); however, it seems that efficacy as a single agent is only moderate.4, 5, 6
JAK2 mutations or fusion proteins leading to constitutive activation of JAK2 have long been known to have a role in MPNs and leukemia.7, 8 JAK2 inhibitors, such as pacritinib (SB1518),9 an oral inhibitor currently in Phase II clinical studies, as well as other JAK2 inhibitors show significant efficacy in treating MPNs,10, 11, 12 reducing the JAK-STAT (signal transducer and activator of transcription) signaling, spleen size, JAK2V617F mutation burden, as well as levels of certain cytokines/growth factors relevant in MPNs. Nuclear JAK2 has been reported to have a second, epigenetic function that might contribute to leukemogenesis.13 The JAK-family kinases were shown to cause phosphorylation of Y41 on histone H3, displacing heterochromatin protein 1α from its position bound to histone H3. Sustained displacement of the heterochromatin protein 1α triggers increased expression of oncogenic transcription factors, such as LMO2, enhanced mitotic recombination, chromosomal disjunction and aneuploidy. All these changes promote oncogenesis and are consistent with the phenotypic consequences observed after constitutive JAK2 activation in hematological malignancies.13, 15
A mutation in the FMS-like tyrosine kinase 3 (FLT3), the FLT3 internal tandem duplication (ITD), causes constitutive active FLT3 signaling, leading to activation of the downstream STAT5. The FLT3–ITDs are described in up to 35% of all AML patients,16, 17 and a single FLT3–ITD is sufficient to induce a myeloproliferative phenotype, as shown in genetic mouse models,18, 19 demonstrating the importance of mutated FLT3 in the pathogenesis of acute leukemia.
The HDACi givinostat (ITF2357) has been reported to reduce levels of total JAK2 as well as STAT5 in the JAK2V617F mutant cells.20 In addition, the HDACi panobinostat and the JAK2/FLT3/RET inhibitor TG101209 are reported to exert synergistic cytotoxic effects against cell lines carrying the JAK2V617F mutation.21 Another interesting recent observation is that HDACi selectively target FLT3–ITD for degradation in AML cells.22 In addition, superior activity on AML cell apoptosis has been reported for a combination of an HDACi and a FLT3 inhibitor.23, 24 On the basis of these encouraging observations, we explored at multiple levels the in vitro and in vivo synergy between the HDACi pracinostat and the JAK2/FLT3 inhibitor pacritinib.
Pracinostat is an oral pan-HDACi with favorable pharmacokinetics25 and good tolerability in patients,26, 27 which is currently explored as a single agent in multiple Phase II clinical studies, for solid tumors as well as myelodysplastic syndrome, AML and myelofibrosis. Pacritinib9 is an oral JAK2/FLT3 kinase inhibitor, also with favorable pharmacokinetics and good tolerability, which is currently in Phase II clinical studies for myelofibrosis and lymphoma.12 The studies described in this manuscript provide a rationale for the combination of these two drugs as a treatment for AML patients, especially those with either mutated FLT3 or JAK2.
Materials and methods
Compounds
Pracinostat (SB939) as hydrochloride salt and pacritinib (SB1518) as citrate salt were synthesized by SAI Advantium Pharma Ltd (Hyderabad, India). For in vitro studies, drugs were dissolved in dimethyl sulfoxide (10 mM stock); for in vivo studies, the dosing solutions for oral gavage were prepared in 0.5% methylcellulose (w/v) and 0.1% Tween-80 in H2O (MC/Tween), stored at 4 °C, and prepared freshly at least every week. All in vivo doses described for pacritinib refer to the free base.
Cells
Cell lines used were obtained from either the American Type Culture Collection (Manassas, VA, USA) or the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). SET-2, KG-1, F36-P, HEL92.1.7, THP-1, MV4-11, MOLM-13, ML-2, ME-1, SH-2, HL-60, MOLM-16, 32D, K562, KARPAS-1106P and RS4;11 cells were all cultivated according to the vendor’s instructions, tested for mycoplasma contamination (Mycoplasma Plus PCR Primer Set, Stratagene; Agilene Technologies Inc., Santa Clara, CA, USA) and verified by STR profiling (John Hopkins University, Baltimore, MD, USA). Granulocyte macrophage colony-stimulating factor to supplement F-36P cell growth medium was obtained from i-DNA Biotechnology (Singapore), fetal bovine serum was obtained from PAA Laboratories GmbH (Pasching, Austria).
Primary AML cells
Peripheral blood mononuclear cells or bone marrow mononuclear cells from AML patients were obtained from AllCells (Emeryville, CA, USA) and ProteoGenex (Culver City, CA, USA) from a total of 16 patients. Cultivation, expansion and analysis were carried out as described previously.28
Cell proliferation assay and determination of in vitro synergy
Cells were seeded in 96-well plates at a predetermined optimal density (in the log phase and rested for 2 h) before drug treatment and then treated for 48 h with drug concentrations from 10 μM to 1.5 nM in 9 serial-dilution steps with 0.1% solvent, and using the CellTiter-Glo Assay (Promega, Madison, WI, USA), according to the manufacturer’s instructions, in a total volume of 100 μl. At least three independent experiments were performed in triplicates. IC50 were determined using XLfit (IDBS, Guildford, UK). To calculate the in vitro synergy of two drugs, they were combined at a constant ratio, based on the individual drug’s IC50 concentrations, with the highest doses used being 8 × IC50 concentrations.29 For sequential treatments, cells were treated with drug 1 for 24 h followed by a 24 h treatment with drug 2; simultaneous treatments were performed for 48 h. Synergy was determined using the CompuSyn software (v2007; ComboSyn Inc., Paramus, NJ, USA). Combinatorial indices (CIs) were calculated29 CI<1 (synergy), CI>1 (antagonism) and CI=1 (additive) for in vitro combinations.
Lysis and western blots
Cell lysis, protein quantification and western blots were performed as described previously.20 Antibodies against pFLT3 Y591 (no. 3461), pSTAT3 Y705 (no. 9145), pJAK2 Y1007/1008 (no. 3776), STAT5 (no. 9310), and cleaved poly-adenosine di-phosphate ribose polymerase N214 (no. 9541) as well as horse-radish peroxidase linked secondary antibodies were from Cell Signaling Technologies (Danvers, MA, USA). The antibodies against pSTAT5 Y694 (no. 611965) and STAT3 (no. 610190) were obtained from BD Biosciences (San Jose, CA, USA). The β-actin antibody (no. 2066) and the FLT3 antibody were from Sigma Aldrich (St Louis, MO, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. The LMO2 antibody (no. 91652) was obtained from Abcam (Cambridge, MA, USA).
Subcutaneous animal models
Female BALB/c nude mice were obtained from the Biological Resource Centre (Biopolis, Singapore), female SCID mice were purchased from BioLASCO (Taipei, Taiwan) and female SCID/Beige mice were obtained from Charles River Laboratories (Willmington, MA, USA). BALB/c nude mice (BALB/cOlaHsd-Foxn1nu) were 8–10 weeks of age; SCID mice (C.B-17/IcrHan™Hsd-Prkdcscid) 5–11 weeks of age, and SCID-Beige mice (C.B-17.Cg-PrkdcscidLystbg/Crl) were 9 weeks old. Standard protocols were followed, in compliance with the National Institutes of Health and National Advisory Committee for Laboratory Animal Research guidelines (IACUC approval no. 0800371).
For subcutaneous (s.c.) AML models, mice were implanted with 5 × 106 cells (or 1 × 107 for MV4–11 cells) in the right flank. Cells were re-suspended in 50 μl serum-free growth medium, mixed 1:1 with Matrigel (Cat no. 354248; BD Bioscience) and injected in a total volume of 100 μl. Tumor volumes were monitored using caliper measurements and volumes calculated using the formula: tumor volume (mm3)=(w2 × l)/2 (w=width and l=length in mm of the tumor xenograft). Pracinostat and pacritinib were administered by per oral gavage in a volume of 10 ml/kg if not otherwise stated. Pracinostat was dosed at 25–125 mg/kg either daily or every other day. Pacritinib was dosed 50–150 mg/kg daily or twice daily. Tumors were excised on the last day of treatment, 3 h post-dose. In vivo synergy was determined using the Clarke’s CI (CCI) Equation:30 CCI=A/B−(C/B × D/B), and must be <0 to be synergistic, where A is the average tumor measurement from the combination group; B, the average measurement from the vehicle control; and C and D, the average measurements from monotherapy 1 and 2, respectively. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
Orthotopic AML model
SCID mice (from Biological Resource Centre) were injected intravenously with 1 × 107 HL-60 cells in 100 μl serum-free medium. Mice were monitored three times per week for signs of paralysis. A separate take-rate control group was analyzed on day (d) 29 as previously described,31 indicating a good take-rate. Treatment of the experimental group started 1 day later, on d30 after inoculation (before the first mouse in this group showed symptoms of paralysis). Blood counts were performed from tail blood, using a ScilVet abc hematology analyzer (Scil Animal Care Company, Gurnee, IL, USA) according to the manufacturer’s instructions. Statistical analyses were performed using GraphPad Prism 5.
Pharmacokinetic analysis of pracinostat and pacritinib
The bioanalysis to detect levels of either pracinostat or pacritinib from 50 μl murine plasma was performed as described previously.9, 20 Two independent experiments were performed, either in 16 weeks old female BALB/c nude mice (BALB/cOlaHsd-Foxn1nu) from Biological Resource Centre (n=3–5 per time point), or in 8–12 weeks old female BALB/c mice (BALB/cOlaHsd) from Biological Resource Centre (n=4 per time point). Pharmacokinetic parameters were calculated by a non-compartmental method using WinNonlin 5.2 software (Pharsight Corp., Sunnyvale, CA, USA).
Cytokine/growth factor analysis
Murine or human plasma samples were analyzed by Millipore Corp. (Billerica, MA, USA) for cytokine/chemokine/growth factor measurements using the Luminex xMAP technology. The Milliplex MAP Mouse Cytokine/Chemokine panel (premixed 32-plex, MPXMCYTO-LIX) was used for mouse samples.
Results
Pracinostat inhibits JAK-STAT as well as FLT3-STAT5 signaling
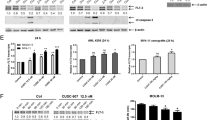
Cells, expressing wild-type (wt) JAK2 (32D murine myeloid cells) or mutant JAK2V617F (HEL92.1.7 and SET-2) were treated with pracinostat in concentrations ranging from 125–500 nM for 24 h, and levels of pJAK2, JAK2, pSTAT5 and STAT5 were measured. Pracinostat treatment decreased both pJAK2 (Y1007/08) and pSTAT5 (Y694) levels, and also total JAK2 and STAT5 protein in JAK2V617F mutant cells (see Figure 1a). These proteins remained unaffected in the JAK2 wt cells 32D and KARPAS 1106-P (Figure 1a right hand panel and data not shown). To determine the effects of pracinostat on cells with either mutated or wt FLT3, MV4-11 cells or MOLM-13 cells carrying FLT3 ITDs were compared with RS4;11 cells with wt FLT3. In FLT3-ITD cell lines, pracinostat led to a near-total ablation of pFLT3 (Y591) at 500 nM in MV4-11 and MOLM-13 cells, respectively, with very steep dose response (Figure 1b). There was a concomitant decrease in total FLT3 as well as pSTAT5, described to be a direct downstream substrate of FLT3 in FLT3-ITD cell lines.32 In RS4;11 cells, which express wt FLT3, levels of pFLT3 and FLT3, and pSTAT5 were also reduced, but to a lesser extent and more gradually with dose than in the FLT3-ITD cell lines. In contrast to the FLT3-ITD cell lines, there was no decrease in total STAT5; however, the levels of total STAT5 are very low.
Pracinostat downregulates JAK and FLT3 signaling in JAK2V617F and FLT-ITD cell lines, and shows synergy in combination with pacritinib. (a–d) Western blot analyses from 25 or 50 μg (pFLT3 and FLT3 blots only) of total cell lysate of the indicated cell lines with JAK2V617F, JAK2, or FLT3-ITD or FLT3 wt are shown. (a, b) Cells were treated with the indicated concentrations of pracinostat (SB939) for 24 h. (c, d) Cells were treated for 24 h with pracinostat with pacritinib (SB1518) added in the indicated concentrations for the last 2 h of incubation before lysis.
Pracinostat and pacritinib show in vitro synergy on STAT signaling and apoptosis
To determine whether pacritinib, a JAK2/FLT3 kinase inhibitor, combined with pracinostat would lead to even more pronounced inhibition of STAT signaling and cause increased cell death in JAK2- or FLT3-driven disease models, JAK2V617F mutant cell lines were treated simultaneously with pracinostat and pacritinib. As described previously, pacritinib treatment alone increased pJAK2 levels in the JAK2V617F cell lines,9 whereas downstream pSTAT5 was decreased. The combination of pacritinib and pracinostat completely abrogated the JAK2-autophosphorylation in Set-2 cells (Figure 1c). The same effect, although to a lesser extent, was observed in HEL92.1.7 cells. Concurrently with the decrease in pSTAT5, there was an increase in cleaved poly-adenosine di-phosphate ribose polymerase levels, indicating enhanced cell death after the combination of pracinostat and pacritinib. This was also more pronounced in SET-2 cells than HEL92.1.7 cells (Figure 1c). The combination also increased efficacy in reducing levels of pFLT3 and total FLT3, as well as increasing cleaved poly-adenosine di-phosphate ribose polymerase in the FLT3-ITD cell lines MV4-11 and MOLM-13 (Figure 1d). There was also a greater reduction in the oncogenic transcription factor LMO2, suggesting that pracinostat and pacritinib also might synergize on the epigenetic level (bottom panel of Figure 1d) in FLT3-ITD cell lines.
Pracinostat potently inhibits proliferation of different AML subtypes as a single agent and is synergistic with pacritinib in JAK2V617F or FLT3-ITD AML cell lines
AML cells are among the most sensitive cancer cells to HDAC inhibition.20 The IC50 on cell proliferation in a panel of 11 AML cell lines ranged from 70–560 nM, with the panel covering most subtypes of AML according to the French–American–British classification, with the exception of M1 and M3 (see Figure 2a). Primary AML cells, expanded from peripheral or bone marrow blasts in the presence of FLT3 ligand, stem cell factor, interleukin (IL)-3 and IL-6 were on average less potently inhibited, with mean IC50 values for the different French-American-British (FAB) classification types ranging between 622 nM and 1.5 μM. Individual blasts had IC50 as low as 169 nM and others as high as 2.2 μM (Figure 2b, Supplementary Figures 1 and 2). Within the small number of cells and cell lines tested, there was no clear trend for any AML subtype to be more sensitive to HDAC inhibition than others. The two FLT3-ITD primary AML blasts (Figure 2b, shaded gray) had similar sensitivity towards pracinostat as the FLT3 wt blasts. For pacritinib, cell lines dependent on either JAK2 or FLT3 signaling (such as SET-2, MV4-11 or MOLM-13) were the most sensitive to JAK2/FLT3 inhibition, both on cell proliferation as well as on the target biomarker levels (described elsewhere9). Similarly, primary AML blasts containing the FLT-3 ITD were amongst the most pacritinib-sensitive cells in cell proliferation assays.33 In vitro synergy was observed when combining pracinostat and pacritinib (i.e., CIs<1) in both FLT3-ITD cell lines and JAK2V617F cell lines, but not in HL-60 or KG-1 cells, which are wt for both genes. However, synergy was observed for F-36P cells, which are wt for both JAK2 and FLT3, but are fully dependent on JAK2 signaling. The best effects were observed in FLT3-ITD cell lines when both drugs were administered simultaneously, with CIs of 0.77 and 0.41 at a combination of doses that effectively blocked 90% of cell proliferation (ED90) for MOLM-13 and MV4-11, respectively (Table 1). Weaker synergy was noted in JAK2V617F cell lines when pracinostat was administered 24 h before pacritinib, with CIs of 0.95 and 0.81 at ED90, respectively, for SET-2 and HEL92.1.7 cells. The addition of pacritinib before pracinostat was antagonistic in all cell lines tested at ED90 (Table 1).
Pracinostat potently inhibits cell proliferation in AML cell lines and primary AML cells. (a) IC50 of cell lines tested in 48 h cell proliferation assays (CellTiterGlo); FAB: French–American–British classification of AML cells. Results show mean±s.d. from at least three rounds of experiments, each performed in triplicates. (b) Primary AML blasts from 16 patients were expanded and then treated on d12–d13 with dimethyl sulfoxide or pracinostat serially diluted in nine steps from 10 μM to 1.5 nM for 48 h. Results show means from two rounds of blast expansion/proliferation assays. Gray-shaded icons depict blast cells carrying the FLT3-ITD.
Pracinostat is efficacious in various animal models of human AML
Pracinostat as a single therapy was tested in different mouse models of human AML. Treatment of mice bearing MV4-11 xenografts with pracinostat (25 or 50 mg/kg per day for 21 days) induced significant (P<0.001) inhibition of tumor growth (TGI), by 59 and 116%, respectively. Complete tumor regression was observed in 6 out of 10 animals at the end of the treatment after the 50-mg dose (Figure 3a). To determine the anti-tumor efficacy in a model that is less sensitive to pracinostat, based on cell proliferation in vitro, mice bearing HEL92.1.7 xenografts were treated with pracinostat at either 75 or 125 mg/kg every other day for a total of 17 days. Dose-dependent TGI (55%) was observed, which was statistically significant (P<0.01) for the higher dose. Pracinostat was very well tolerated in both studies (maximum body weight losses of 7 and 4.7%, respectively, in the MV4-11 and HEL92.1.7 model). See Supplementary Table 1 for an overview of the efficacy in different models. To assess the efficacy of pracinostat in a more physiological relevant model, HL-60 cells were engrafted orthotopically (via tail vein injection). Treatment with 125 mg/kg pracinostat in a thrice weekly schedule was started once the disease was established on d30, (as measured by fluorescence-activated cell sorting analysis of human cell-surface markers on HL-60 cells in the murine bone marrow and peripheral blood, data not shown), but before the onset of the first symptoms, such as hind-leg paralysis. Pracinostat treatment led to a 17-day delay of the disease onset and a 50% reduction of death caused by progressive AML on study d24 (Figure 3c, upper panel). White blood counts on d15 of the study were six-fold elevated and lymphocyte counts three-fold elevated in vehicle-treated animals compared with disease-free SCID mice (i.e., mice that were not inoculated with HL-60 cells). Pracinostat treatment led to a significant (P<0.001) decrease in blood counts, effectively normalizing blood counts to the levels of disease-free mice, without significantly reducing the numbers of platelets at the same time. These data demonstrated the efficacy and good tolerability of pracinostat in an orthotopic model of AML (Figure 3d).
Pracinostat is efficacious in two s.c. and an orthotopic model of AML. (a) Female BALB/c nude mice (n=10 per group) were inoculated with 1 × 107 MV4-11 cells s.c. into the right flank, and treatment as indicated was started on study d9. (b) Female BALB/c nude mice (n=5 per group) were inoculated s.c. with 5 × 106 HEL92.1.7 cells, treatment was started on d18. (c) Female SCID mice (n=6 per group) were injected with 1 × 107 HL-60 cells intravenously and scored daily for symptoms of paralysis. Treatment with pracinostat (SB939) 125 mg/kg three times per week was initiated on d30, before the first animal showed symptoms of paralysis. Daily disease scores are shown in (c); blood counts of naive mice, vehicle- or pracinostat-treated mice are shown in (d). ‘Naive mice’ refers to age-matched SCID mice that were not injected with HL-60 cells. Statistical significance was determined using analysis of variance/Bonferroni, ***P<0.001 and **P<0.01 significance compared with vehicle-treated animals.
Pracinostat and pacritinib are synergistic in two different AML disease models
The SET-2 model of megakaryoblastic leukemia was chosen to test the in vivo combination of pacritinib and pracinostat, as the growth of this cell line has been shown to be dependent on the JAK2-STAT5 signaling.34 Pacritinib as a monotherapy, dosed at 150 mg/kg twice daily (i.e., the maximum tolerated dose) led to a 61% TGI, whereas pracinostat dosed at 75 mg/kg q.o.d (less than half the maximum tolerated dose) inhibited tumor growth by 56%. On the basis of tumor volume, the TGI of the drug combination was 86% (Figure 4a). On the basis of tumor weight, monotherapy led to 47 and 42.5% TGI for pacritinib or pracinostat, respectively (Figure 4b), whereas the combination TGI by 75%, giving rise to a CCI value of −0.06 (indicating synergy). Interestingly, several infrequent, but obvious metastases, were found in the lymph nodes of mice in each group, except in mice dosed with pacritinib alone or the animals treated with the combination of both drugs (data not shown). When analyzing tumors at the end of the study after chronic treatment with either monotherapy or the drug combination, pSTAT5 levels were decreased by about 50% by pacritinib alone and by 75% by the combination treatment. Pracinostat alone led to increased pSTAT5 levels compared with vehicle treated animals (Supplementary Figure S3). Subsequently, the combination of pracinostat and pacritinib was also tested in mice bearing FLT3-ITD-driven MOLM-13 s.c. xenografts. In vitro data showed that the MOLM-13 cells were the most sensitive to both pracinostat and pacritinib,9 and that the synergy of combination treatment was greater than in SET-2 cells (Table 1, Figure 2a). Therefore, a lower dose of pacritinib was used (50 mg/kg daily) in combination with pracinostat (75 mg/kg q.o.d) for the in vivo study with MOLM-13. This MOLM-13 xenograft model is extremely aggressive, within 10 days after cell injection when treatment was initiated, with mean tumor volumes being 553 mm3. Tumors were excised on d7 of the study (after 8 days of treatment) when the mean tumor volume in the vehicle group was 2952, mm3 (and the average tumor weight was 1770, mg). Treatment with pracinostat or pacritinib as a monotherapy led to a 19.2 and 14.7% reduction in tumor weight, respectively, whereas the combination treatment reduced the average tumor weight by 46.9%, to 940 mg (P<0.001) (Figure 4c), giving rise to a synergistic CCI of −0.16. Western blot analysis of the tumors (harvested 3 h post pacritinib treatment) on d7 showed that both pacritinib and, to a lesser extent, also pracinostat decreased levels of pSTAT5. Levels of pFLT3 were only decreased marginally, but chronic treatment with pacritinib alone increased expression total levels of FLT3 significantly. In combination, pacritinib and pracinostat not only normalized FLT3 levels, but also reduced pSTAT5 levels and nearly abolished all pFLT3 (Figure 4d).
Pracinostat combined with pacritinib is efficacious and synergistic in vivo in two different models of human AML. Female SCID-beige mice (n=12 per group) were inoculated with 5 × 106 SET-2 cells and treatment was started on d33 post-implantation (a, b). For the combination group, both pacritinib (SB1518) and pracinostat (SB939) were administered in half the volume (as a 20 mg/kg solution) every other day; when both compounds were given simultaneously, all other doses were given at 10 mg/kg, at least 8 h apart for 19 days (d0–d18). There was one non-treatment-related death in the vehicle group on study d11 (gavaging error). Female SCID mice (n=12 per group) were inoculated with 5 × 106 Molm-13 cells (c, d). Treatment was started on d11, animals were dosed every other day for 8 days, in contrast to (a), animals were dosed with pacritinib only daily (in the evenings), pracinostat dosing remained unchanged, all dosing was done in a 10 mg/kg solution. On the last day, mice were killed and excised tumors were weighed. The doses showing significant TGI versus vehicle (on the last day of the study) using analysis of variance/Dunnet’s post test, are indicated with **P<0.01 or ***P<0.001. (e) Tumors from the MOLM-13 efficacy study (c, d) were harvested on the last day 3 h post pacritinib dosing and immediately lysed. Western blot analyses for pFLT3, FLT3, pSTAT5 and β-actin are shown from the lysates of three randomly selected tumors from each treatment group of (c).
Many of the s.c. implanted tumors in this model metastasized (to the lungs, the nearest breast fat-pad and/or the lymph nodes). In the vehicle group, 9/12 mice had 1 or 2 metastases, some of them weighing as much as 208 mg. Metastatic incidences (i.e., obvious, individual metastases per mouse) were reduced by 60 and 50%, respectively, after single-agent treatment with pacritinib or pracinostat, respectively (Figure 4e). In combination, metastatic incidents were reduced by 90%, leading to a CCI of −0.1, indicating that the combination was able to inhibit metastatic spread synergistically. The pharmacokinetic parameters of pracinostat were similar after 14 days of co-administration with pacritinib, with the concentration–time curve being nearly identical on d1 and d14 (Figure 5a). The Cmax and AUC0-∞ of pacritinib were increased approximately two-fold in Cmax and approximately four-fold in AUC after chronic dosing in combination (Figure 5b). Pacritinib also exhibited a two-fold increase in exposure following repeated dosing in toxicokinetic studies in mice as single agent (data not shown). Therefore, the observed in vivo synergy is unlikely because of a moderate increase in pacritinib exposure in the combination study design.
Pharmacokinetic analysis of pracinostat and pacritinib administered in combination. Female BALB/c nude mice were dosed with 150 mg/kg pacritinib (SB1518) plus 75 mg/kg pracinostat (SB939) simultaneously either once (‘day 1’) or for a total of 14 days (i.e., 7 doses of SB939, administered every other day in the morning, plus SB1518 for 14 times, every day morning and evening, ‘day 14’). Plasma was collected at 10, 30 and 60 min post-dose on d1/d14 (n=5) as well as 4, 8 and 24 h post-dose (n=3). Pharmacokinetic parameters were calculated by a non-compartmental method, using WinNonlin software, and compared with values obtained in previous experiments in the same mouse strain. * Indicates that values were extrapolated from BALB/c nude mice dosed with 50 mg/kg pacritinib or pracinostat. Results for pracinostat are shown in (a), and for pacritinib in (b).
Effects of pracinostat, pacritinib or the combination on tumor-induced growth factors and cytokines/chemokines
HDACi as well as JAK2 inhibitors have been described to affect the production of various growth factors, chemokines and cytokines,35, 36, 37, 38 thereby influencing tumor growth. Plasma cytokine/growth factor/chemokine levels in naive mice (without SET-2 xenograft), or xenografted mice treated with vehicle, pracinostat or pacritinib alone, or a combination thereof, were analyzed using multiplex analysis/enzyme-linked immunosorbent assay. SET-2 tumors caused the plasma levels of IL-6, IP-10, KC, MCP-1 and MIP-1β to increase (Figure 6) compared with levels present in naive mice (i.e., mice that were not inoculated with tumor cells). Single-agent treatment with pacritinib or pracinostat led to the normalization of IL-6, IP-10, KC and MIP-1α. A synergistic effect for the combination of pacritinib or pracinostat was observed for the chemokine MCP-1. Plasma levels of MCP-1 were elevated from 44 (in naive mice) to 168 pg/ml in tumor-bearing mice. Pracinostat or pacritinib as single agents reduced levels by 40 and 10%, respectively. In combination, MCP-1 levels were reduced by 69% (to 53 pg/ml).
Pracinostat and pacritinib have synergistic effects on AML-induced plasma cytokines/growth factors/chemokines. Plasma levels of a panel of 32 cytokines/growth factors/chemokines were analyzed using the MAP Mouse Cytokine/Chemokine panel from Millipore. Plasma was collected from SCID-beige mice bearing s.c. SET-2 tumors on d18, 3 h post-dose, after chronic dosing with pacritinib and pracinostat from the mice used in the efficacy study described in Figure 4.
Discussion
In this study, we show the efficacy and tolerability of the pan HDACi pracinostat in various in vitro and in vivo models of AML, and show synergistic effects at multiple levels in combination with the JAK2/FLT-3 inhibitor pacritinib in both the in vitro and in vivo setting. We also explored the possible mechanisms for these synergistic anti-tumor effects. Previously reported synergistic effects of an HDACi combined with a JAK2 inhibitor was attributed to impaired chaperone function of heat shock protein 90 by the HDACi, promoting proteasomal degradation and depleting total JAK2 levels.21 FLT3 is another heat shock protein 90 client protein, where mutant forms such as FLT3-ITD are more dependent on the chaperone association than their wt counterpart.39 In addition, earlier studies have shown that HDAC inhibition depleted mRNA levels of JAK2V617F.21 Therefore, not surprisingly, we have demonstrated that pracinostat not only reduced JAK2/STAT5 protein levels in cells bearing a JAK2 mutation, but also FLT3/STAT5 levels in cells with a FLT-3 mutations (as seen in Figure 1). Recent studies with the HDACi trichostatin A demonstrate that JAK2/STAT3 signaling was decreased by upregulation of the expression of suppressors of cytokine signaling (SOCS) 1 and 3 genes.40 A possible direct effect of the HDAC inhibition on the phosphorylation of JAK2, STAT5 and FLT3 was not investigated in this study.
SB939 showed potent inhibition predominantly in JAK2V617F- or FLT3-ITD-harboring cell lines (Figure 2a). The cell line with the lowest IC50 of 70 nM was HL-60, which carries an N-RAS mutation. HDACi have been shown to block Ras-dependent signaling and growth transformation.41 Surprisingly, in HEL92.1.7 and MOLM-13 cells, the pracinostat IC50 on proliferation is lower than the IC50 on inhibition of JAK2 or FLT3-ITD protein levels, respectively. This discrepancy might be a result of modulation of other genes besides JAK2V617F and FLT3-ITD by HDAC inhibition.
Pacritinib is an equipotent inhibitor of JAK2 and FLT3, which is effective in reducing JAK2/STAT5 and FLT-3 JAK2 signaling in JAK2 and FLT3 mutant cells, respectively.33 The combination of pracinostat and pacritinib led to synergistic effects with a complete inhibition of downstream STAT5 signaling, an increased efficacy on cell proliferation and the induction of apoptosis. In vitro combination studies in different cell lines with either wt or mutant JAK2 or FLT3 also demonstrated synergy, mostly in cells that carried the mutant protein. One exception was the F36-P cell line. The growth of this cell line depends on exogenously added granulocyte macrophage colony-stimulating factor,42 which signals exclusively via JAK2, making it a JAK2 wt-dependent cell line. This indicates that synergy between a JAK2 inhibitor and an HDACi might also work in cells that are fully dependent on JAK2 (wt) signaling. Consistent with this, similar in vitro synergy was observed in the JAK2 wt SET-2 cells and F36-P cells but not in FLT3 mutant cell lines with the specific pan-JAK inhibitor ruxolitinib in combination with pracinostat (data not shown).
LMO2 is a transcription factor involved in normal hematopoiesis, but also leukemogenesis that is overexpressed in many AML cells.43 Interestingly, LMO2 levels were downregulated synergistically in MOLM-13 cells with pacritinib and pracinostat, and may be a result of another synergistic interaction between JAK2 and HDAC. Dawson et al.43 demonstrate that JAK2 inhibition leads to lower levels of histone H3 Y41 phosphorylation on the promoter of LMO2, whereas increasing the binding of heterochromatin protein 1α at the same site, resulting in lower expression of LMO2. JAK2 may have an epigenetic role in the nucleus to influence the status of H3 acetylation. It has been demonstrated previously that phosphorylation of H3 (on S10) leads to increased efficiency of a subsequent H3 acetylation, resulting in synergistic modifications of gene expression.44
Pacritinib, as well as targeting JAK2, is a potent FLT3 inhibitor. Our group has recently discovered that treatment of FLT3-ITD cells with FLT3 inhibitors lacking JAK2 activity (e.g., ABT-869, VX-680 or sunitinib), leads to an upregulation of JAK2 activity, causing secondary resistance.33 Therefore, although combinations of FLT3 inhibitors and HDACi have been described to show synergy in vitro,23, 24 this combination without the additional JAK2 inhibition could lead to resistance after chronic dosing and not show enhanced efficacy in the in vivo setting. This may explain why none of the studies showing in vitro synergy reported any in vivo synergy data. Pacritinib as a dual JAK2/FLT-3 inhibitor is therefore ideally suited for a combination with an HDACi and superior to an inhibitor that only affects FLT3 kinase without targeting other JAK family kinases.
Although the combination of pracinostat and pacritinib showed synergy in vitro, the synergy was greater in the in vivo setting in both AML models tested. This indicates that there are additional synergistic mechanisms that are only working in the whole animal setting. One example is the synergistic effects observed on metastases. In AML patients, leukemia cutis and extramedullary involvement of organs such as the lungs are common. Respiratory distress syndrome secondary to lung involvement causes a significant percentage of the morbidity/mortality associated with AML.45 Therefore, the synergy observed in reducing metastatic sites in the animal model is certainly of great relevance for AML patients. Interestingly, significantly higher plasma levels of MCP-1 were measured in untreated AML patients with extramedullary sites involved than in those with complete remission.46 This highlights the potential therapeutic benefit with our observation that pracinostat and pacritinib synergistically decrease MCP-1 plasma levels as well as metastatic occurrences. In both models tested, chronic treatment with one drug alone led to the increase of a signaling pathway. Pacritinib in the Molm-13 model or pracinostat (Set-2 model) led to increased FLT3 or pSTAT5 levels, respectively, whereas the combination treatment in both studies was most efficiently suppressing the signaling, indicating that a combination treatment can overcome treatment-induced resistance.
Effects of tumor-induced elevations of cytokine and chemokine levels may be another mechanism for the synergy observed with pracinostat and pacritinib. HDACi as well as JAK2 inhibitors have been described to affect the production of various growth factors and cytokines,35, 36, 37 thereby influencing tumor growth. Manshouri et al.38 recently showed that resistance to JAK2-inhibitor treatment of MPNs is mediated by cytokines produced by the bone marrow stroma. Distinctly high levels of IL-6, FGF4 and CXCL10/IP-10 were detected in co-cultures of stromal cells and SET-2 cells, mediating resistance to the JAK2 inhibitor antiprimod. Although SCID beige mice that lack B-cells, T-cells, as well as natural killer cells, were used for the SET-2 AML model in our studies, high circulating levels IL-6, IP-10, KC and MCP-1 were measured in the tumor-bearing mice without drug treatment. Treatment with pacritinib or pracinostat as single agents led to the normalization of IL-6, IP-10, KC and MIP-1α, and a synergistic normalization of MCP-1 levels was observed with the combination treatment.
Taken together, our studies demonstrated the synergistic efficacy of a combination of pracinostat and pacritinib in in vitro and in vivo models of AML and offer mechanistic insights for this synergy. These data provide a scientific rationale for the combination of pracinostat and pacritinib for advanced acute leukemia, which warrants further exploration in a clinical trial.
References
Quintas-Cardama A, Santos FP, Garcia-Manero G . Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia 2011; 25: 226–235.
Marks PA, Richon VM, Breslow R, Rifkind RA . Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol 2001; 13: 477–483.
Glozak MA, Sengupta N, Zhang X, Seto E . Acetylation and deacetylation of non-histone proteins. Gene 2005; 363: 15–23.
Prebet T, Vey N . Vorinostat in acute myeloid leukemia and myelodysplastic syndromes. Expert Opin Invest Drugs 2011; 20: 287–295.
Mithraprabhu S, Grigoriadis G, Khong T, Spencer A . Deactylase inhibition in myeloproliferative neoplasms. Invest New Drugs 2010; 28(Suppl 1): S50–S57.
Garcia-Manero G, Chuah C, Wilding G, Chang J, Verstovsek S, Faderl S et al. Phase I study of the oral histone deacetylase inhibitor SB939 in patients with advanced hematologic malignancies. Blood 2010; 116 (abstract no. 3292).
Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffé M et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 1997; 278: 1309–1312.
Rawlings JS, Rosler KM, Harrison DA . The JAK/STAT signaling pathway. J Cell Sci 2004; 117: 1281–1283.
Hart S, Goh KC, Novotny-Diermayr V, Hu CY, Hentze H, Tan YC et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia 2011; 25: 1751–1759.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Verstovsek S, Deeg HJ, Odenike O, Zhu J, Kantarjian H, Estrov Z et al. Phase 1/2 study of SB1518, a novel JAK2/FLT3 inhibitor, in the treatment of primary myelofibrosis. Blood 2010; 116 (abstract no. 3082).
Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 2009; 461: 819–822.
He J, Zhang Y . Janus kinase 2: an epigenetic 'writer' that activates leukemogenic genes. J Mol Cell Biol 2010; 2: 231–233.
Plo I, Nakatake M, Malivert L, de Villartay JP, Giraudier S, Villeval JL et al. JAK2 stimulates homologous recombination and genetic instability: potential implication in the heterogeneity of myeloproliferative disorders. Blood 2008; 112: 1402–1412.
Kiyoi H, Naoe T . FLT3 mutations in acute myeloid leukemia. Methods Mol Med 2006; 125: 189–197.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000; 96: 3907–3914.
Li L, Piloto O, Nguyen HB, Greenberg K, Takamiya K, Racke F et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood 2008; 111: 3849–3858.
Lee BH, Williams IR, Anastasiadou E, Boulton CL, Joseph SW, Amaral SM et al. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene 2005; 24: 7882–7892.
Guerini V, Barbui V, Spinelli O, Salvi A, Dellacasa C, Carobbio A et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F). Leukemia 2008; 22: 740–747.
Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R et al. Co-treatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplasm cells. Blood 2009; 114: 5024–5033.
Buchwald M, Pietschmann K, Muller JP, Bohmer FD, Heinzel T, Kramer OH . Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia 2010; 24: 1412–1421.
Bali P, George P, Cohen P, Tao J, Guo F, Sigua C et al. Superior activity of the combination of histone deacetylase inhibitor LAQ824 and the FLT-3 kinase inhibitor PKC412 against human acute myelogenous leukemia cells with mutant FLT-3. Clin Cancer Res 2004; 10: 4991–4997.
Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood 2009; 113: 4052–4062.
Novotny-Diermayr V, Sangthongpitag K, Hu CY, Wu X, Sausgruber N, Yeo P et al. SB939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol Cancer Ther 2010; 9: 642–652.
Yong WP, Goh BC, Novotny-Diermayr V, Otheris O, Goh A, Ethirajulu K et al. A phase I dose escalation study of oral SB939 when administered thrice weekly (every other day) for 3 weeks in a 4-week cycle in patients with advanced solid malignancies. Eur J Cancer 2008; 6 (abstract no. 413).
Razak ARA, Hotte SH, Siu LL, Chen EX, Hirte HW, Powers J et al. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br J Cancer 2011; 104: 756–762.
Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia 2012; 26: 236–243.
Chou TC . Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70: 440–446.
Clarke R . Issues in experimental design and endpoint analysis in the study of experimental cytotoxic agents in vivo in breast cancer and other models. Breast Cancer Res Treat 1997; 46: 255–278.
Novotny-Diermayr V, Sausgruber N, Loh YK, Pasha MK, Jayaraman R, Hentze H et al. Pharmacodynamic evaluation of the target efficacy of SB939, an oral HDAC inhibitor with selectivity for tumor tissue. Mol Cancer Ther 2011; 10: 1207–1217.
Choudhary C, Brandts C, Schwable J, Tickenbrock L, Sargin B, Ueker A et al. Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood 2007; 110: 370–374.
Hart S, Goh KC, Novotny-Diermayr V, Tan YC, Madan B, Amalini C et al. Pacritinib (SB1518), a JAK2/FLT3 inhibitor for the treatment of acute myeloid leukemia. Blood Cancer J 2011; 104: 1079–1084.
Baffert F, Regnier CH, De Pover A, Pissot-Soldermann C, Tavares GA, Blasco F et al. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol Cancer Ther 2010; 9: 1945–1955.
Leoni F, Fossati G, Lewis EC, Lee JK, Porro G, Pagani P et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med 2005; 11: 1–15.
Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood 2008; 112: 1424–1433.
Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 2010; 115: 5232–5240.
Manshouri T, Estrov Z, Quintas-Cardama A, Burger J, Zhang Y, Livun A et al. Bone marrow stroma-secreted cytokines protect JAK2V617F-Mutated cells from the effects of a JAK2 inhibitor. Cancer Res 2011; 71: 3831–3840.
Oshikawa G, Nagao T, Wu N, Kurosu T, Miura O . c-Cbl and Cbl-b mediate 17-AAG-induced degradation of autophosphorylated Flt3-ITD through the ubiquitin proteasome pathway. J Biol Chem 2011; 286: 30263–30273.
Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT et al. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol Carcinog 2012; 51: 174–184.
Hanker AB, Healy KD, Nichols J, Der CJ . Romidepsin inhibits Ras-dependent growth transformation of NIH 3T3 fibroblasts and RIE-1 epithelial cells independently of Ras signaling inhibition. 2009 J Mol Signal 4: 5.
Chiba S, Takaku F, Tange T, Shibuya K, Misawa C, Sasaki K et al. Establishment and erythroid differentiation of a cytokine-dependent human leukemic cell line F-36: a parental line requiring granulocyte- macrophage colony-stimulating factor or interleukin-3, and a subline requiring erythropoietin. Blood 1991; 78: 2261–2268.
Cobanoglu U, Sonmez M, Ozbas HM, Erkut N, Can G . The expression of LMO2 protein in acute B-cell and myeloid leukemia. Hematology 15: 132–134.
Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD . Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 2000; 5: 905–915.
Stefanidakis M, Karjalainen K, Jaalouk DE, Gahmberg CG, O'Brien S, Pasqualini R et al. Role of leukemia cell invadosome in extramedullary infiltration. Blood 2009; 114: 3008–3017.
Mazur G, Wróbel T, Butrym A, Kapelko-Slowik K, Poreba P, Kuliczkowski K . Increased monocyte chemoattractant protein 1 (MCP-1/CCL-2) serum level in acute myeloid leukemia. Neoplasma 2007; 54: 285–289.
Acknowledgements
We are grateful to Yung Kiang Loh and Nina Sausgruber for their technical help during the animal experiments and for western blots, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Novotny-Diermayr, V., Hart, S., Goh, K. et al. The oral HDAC inhibitor pracinostat (SB939) is efficacious and synergistic with the JAK2 inhibitor pacritinib (SB1518) in preclinical models of AML. Blood Cancer Journal 2, e69 (2012). https://doi.org/10.1038/bcj.2012.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2012.14
Keywords
This article is cited by
-
Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies
Signal Transduction and Targeted Therapy (2023)
-
The novel histone deacetylase inhibitor pracinostat suppresses the malignant phenotype in human glioma
Molecular Biology Reports (2022)
-
Characterizing binding intensity and energetic features of histone deacetylase inhibitor pracinostat towards class I HDAC isozymes through futuristic drug designing strategy
In Silico Pharmacology (2021)
-
Aktuelle Entwicklungen in der Therapie der AML
InFo Hämatologie + Onkologie (2019)
-
Emerging therapies for acute myeloid leukemia
Journal of Hematology & Oncology (2017)