Abstract
Background:
Carcinoma of unknown primary (CUP) is a clinical presentation with a poor prognosis. Inflammation-based prognostic systems are stage-independent prognostic predictors in various malignancies. We aimed to assess the accuracy of the modified Glasgow Prognostic Score (mGPS), neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) as objective prognostic models in CUP.
Methods:
We derived inflammatory scores in 60 consecutive CUP referrals to the Imperial College oncology unit between 1996 and 2011. Patient demographics, treatment and staging data and full blood profiles were collected. An independent cohort of 179 patients presenting to the Taipei Veterens Hospital between 2000 and 2009 were used as a ‘validation’ data set. Uni- and multivariate survival analysis was used to predict the overall survival (OS).
Results:
Sixty patients were included: median age 61 (range: 33–86); 51% men; median OS 5.9 months (0.7–42.9); 88% with distant metastases. On univariate analysis NLR >5 (P=0.04) and mGPS (score 1–2) (P=0.03) correlated with OS. Multivariate analysis demonstrated significant hazard ratios for NLR; 2.02 (CI 1.0–4.1) (P=0.04) and mGPS; 1.52 (CI 1.0–2.3) (P=0.03). These findings were reinforced by analysis of the validation data.
Conclusion:
NLR and mGPS are independent, externally validated prognostic markers in CUP, with superior objectivity compared with performance status.
Similar content being viewed by others
Main
Despite advancements in the diagnosis of cancer, in up to 5% of patients with a histologically confirmed diagnosis of metastatic malignancy, the primary site cannot be identified despite adequate pre-treatment investigation (Pavlidis and Fizazi, 2009). Carcinoma of unknown primary (CUP) includes a heterogeneous population of patients, many of whom will present with a short history of symptoms associated with the presence of metastatic disease (Mayordomo et al, 1993). The natural history of the majority of CUP cases is invariably characterised by an aggressive clinical course with median survival times rarely exceeding 6 months from diagnosis (Pavlidis et al, 2012). The role of systemic treatment is particularly controversial in CUP as no specific chemotherapy regimen—whether taxane, platinum or anthracycline based – has been proven to extend patients’ survival (Golfinopoulos et al, 2009). Moreover, the significant side-effect profile resulting from the combination of multiple cytotoxic agents may reduce the intended benefits of treatment such that a significant number of patients will experience toxicity without any clinical benefit. As the main goal of chemotherapy in this population is essentially palliation, patient selection is important.
Although discrete clinical phenotypes with limited stage nodal disease or neuroendocrine differentiation may exhibit better response to treatment and favourable survival outcomes, 80% of patients cluster into a heterogeneous ‘poor prognostic’ group for which a unifying staging system does not exist (Pavlidis, 2012).
In addition, there is a lack of validated prognostic biomarkers in CUP hampering guidance on treatment allocation and estimation of survival benefit from treatment. A number of clinical and laboratory parameters have been considered for their prognostic significance in CUP. Previous work from Penel et al (2009) suggests that the performance status (PS), LDH, albumin and number of metastatic sites are useful predictors of mortality. In more recent literature, analysis of 311 CUP patients reinforced the prognostic value of PS, clinicopathologic subgroup, as well as a role for leukocytosis as independent negative prognostic indicators which were utilised to form a prognostic algorithm (Petrakis et al, 2013).
The Eastern Cooperative Oncology Group PS is widely used as a semi-quantitative scale reflecting the symptom burden of patients with cancer, with implications in terms of treatment allocation (Oken et al, 1982). However, PS is a subjective measure and therefore potentially open to variance and bias, with only moderate concordance observed between oncologists and patients (Blagden et al, 2003). There is therefore, a clear need for a more accurate, reliable prognostic score for patients with CUP that can be utilised in routine clinical practice.
The association between weight loss, poor PS, disease progression and poor prognosis in malignancy is well documented (Fearon et al, 2006). There is a considerable body of evidence to suggest that this negative spiral is driven by tumour-related systemic inflammation, resulting in a release of pro-inflammatory cytokines such as IL-1β, IL-6 and TNFα (Esper and Harb, 2005). Understanding the role of systemic inflammation in cancer pathogenesis has been a key in the development of novel and more objective prognostic markers. The modified Glasgow Prognostic Score (mGPS), which incorporates C-reactive protein (CRP) and serum albumin, has been validated in multiple tumour types including gastrointestinal, lung and renal as negative prognostic marker. The extent of research into the prognostic value of mGPS is best summarised in a recent literature review of the topic (McMillan, 2013). In addition, the neutrophil–lymphocyte ratio (NLR) and the platelet–lymphocyte ratio (PLR) have been shown to correlate with the survival (Walsh et al, 2005; Guthrie et al, 2013).
The aims of the current study were to assess the clinical utility of inflammation-based scores (GPS, NLR and PLR) in two independent cohorts of patients diagnosed with CUP and to compare their accuracy with that of established prognostic markers including PS.
Materials and Methods
Sixty consecutive patients with a diagnosis of CUP who attended the Oncology Department, Hammersmith Hospital between 1996 and 2011, were used to represent a training data set. The diagnosis of CUP fulfilled the criteria of the European Society of Medical Oncology clinical practice guidelines (Fizazi et al, 2011). All clinical variables were determined at the time of referral to the unit. The mGPS was calculated as described previously (McMillan, 2008). Briefly, patients with both a normal albumin (>35 g l−1) and CRP (<10 mg dl−1) were allocated a score of 0. Patients in whom only one of these abnormalities was present were allocated a score of 1, whereas those with both abnormal CRP and albumin were given a score of 2. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The NLR⩾5 was considered elevated as previously described. The same calculation was applied to derive the PLR, with 300 being the cutoff for positivity, in accordance with the previously published literature (Walsh et al, 2005; Smith et al, 2009).
In addition, we evaluated the significance of the tested prognostic models by means of an independent validation set in a separate cohort of 179 patients with similar characteristics from the Division of Haematology and Oncology at the Taipei Veterans Hospital (Taiwan) (Chen et al, 2012). The study was approved by the local Research Ethics Committee at both sites.
Statistical analysis
Pearson χ2-square test and analysis of variance were used to determine any associations between the variables. Univariate analysis was performed to test the parameters for potential survival benefit using Kaplan–Meier statistics and Log rank testing. Multivariate analysis was performed to delineate any independent variables. A Cox regression analysis with stepwise backward procedure was used to calculate hazard ratios (HRs). Variables with a P value of greater than 0.05 were excluded. The concordance index (c index) was used to rank the prognostic indices in order of best discriminator of patient outcome. The Frank Harrell RMS packages were used to identify a subgroup of predictors by backward elimination. We validated the C statistic by a similar bootstrap procedure using re-sampled data, using 150 iterations (Harrell, 2010). A combined score was derived using logistic regression and predicted probabilities. ROC analysis was subsequently undertaken to derive a resulting C statistic. All statistical analysis was conducted using SPSS statistical package 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Demographics
The clinico-pathologic features of the training set are demonstrated in Table 1. The median age of the patients at baseline was 61 years (range 33–86). A significant proportion of patients had more than one site of metastatic disease (47%), the commonest site being the liver (45%). Almost all of the patients received at least one line of chemotherapy (93%). At the time of analysis, 56 patients had died with an overall median survival of 5.9 months (range 0.7–42.9). Four patients under active surveillance had a median follow-up time of 10.2 months (range 3.8–29.9).
Relationship between Inflammatory scores and patient characteristics
In the training set, a deranged albumin and CRP was present in 57% and 38% of the patients, respectively. An abnormal mGPS (>0) was present in 55% of the patients. A minority of patients had a PLR⩾300 (23%) or an NLR⩾5 (42%). The relationship between the inflammatory scores and clinico-pathological features of the studied patient cohort are summarised in Table 2.
Impact of inflammatory scores as predictors of survival
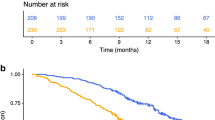
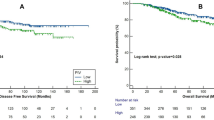
On univariate analysis, significant predictors of overall survival (OS) were NLR (P=0.04), mGPS (P=0.03) and PS (P<0.001) (Table 3)(Figures 1, 2, 3). No association was observed between PLR and survival. Patients with an NLR⩾5 had a median survival of 4 months compared with 8.3 months in patients with an NLR <5. Patients with a mGPS of 0 had median survival of 13.9 months, compared with 5.6 months of mGPS 1 and 3.8 months of mGPS 2. In terms of PS, patients with a PS ⩽2 has a median survival of 12 months, whereas those with a PS >3 had a median survival of 5 months.
Multivariate analysis identified both mGPS (HR 1.53 95% CI 1.03–2.25, P=0.03) and NLR (HR 2.01 95% CI 1–4.09, P=0.04) as significant independent predictors of OS in CUP.
Validation of prognostic models
The NLR and GPS were further assessed for their prognostic power and discriminative ability in an independent and larger validation set. No significant differences were observed on comparison of patients’ clinico-pathological features across the two study population (Table 1). Both the NLR (median survival 11.1 vs 3.5 months; P<0.01) and mGPS (median survival 7.7 vs 3.0 vs 1.8 months for mGPS 0, 1 and 2, respectively; P <0.01) remained significant predictors of OS on univariate analysis, mGPS HR 2.16 (95% CI 1.52–3.08); P<0.01 and NLR HR 2.46 (95% CI 1.66–3.66); P<0.01. A combined prognostic score using both NLR and mGPS was then derived using logistic regression to determine the predicted probability of death. A model for predicted probability of death was determined to be calculated by the equation 1/(1+exp(−f)), where f=−0.3357+(06043*mGPS)+(0.6147*NLR). Subsequent ROC analysis resulted in a C statistic of 0.65 (95% CI 0.55–0.75).
In addition, 66% of the validation population received palliative chemotherapy. Regimes were predominantly cisplatin-based (75.4%), with 43% receiving additional 5-fluorouracil (5-FU) and 11% being taxane based. The treated cohort were significantly younger (mean age 65.5 vs 74.4 years, P<0.01) and had a better PS (P<0.01). The results suggest a survival benefit in those patients who received palliative chemotherapy compared with those who did not (9.2 months vs 1.6 months P<0.01). In addition to this, evaluation of mGPS (median survival mGPS 0; 23.3 vs 4.6 months, mGPS 1; 12.8 vs 2.1 months and mGPS 2; 2.9 vs 0.4 months P<0.01) and NLR (median survival NLR <5 10.2 vs 7 months and NLR >5 21.2 vs 3.5 months P<0.01) correlates with improved prognosis in the treated group (Table 4).
Discussion
In this study, we have validated the use of inflammation-based prognostic scores in CUP. In particular, we have shown mGPS and NLR to be independent predictors of survival in both a training and independent validation set. These values are derived from routinely measured parameters and the results are consistent with previous studies identifying inflammation-based prognostic indices as predictors of outcome in a wide range of malignancies (Forrest et al, 2004; Walsh et al, 2005; Ramsey et al, 2007; Crumley et al, 2008; Smith et al, 2009; Proctor et al, 2011). More recently, Petrakis et al (2013) described a prognostic algorithm constructed from independent markers of poor prognosis in patients with a diagnosis of CUP. The authors identified leukocytosis as an independent negative prognostic factor, supporting our findings. However, the validation of the subsequent algorithm was not carried out in an independent data set.
In univariate analysis, we have shown that PS is a predictor of OS in CUP, as per previously published studies; however, this was not borne out on multivariate analysis (Seve et al, 2006b; Trivanovic et al, 2009; Kodaira et al, 2010; Chen et al, 2012). Although PS is a commonly used tool in clinical assessment of patients undergoing therapy, it is subjective and is vulnerable to bias, with oncologists being comparatively optimistic in their assessment (Ando et al, 2001).
Moreover, we show that inflammation-based scores are significant predictors of outcome across both treated and untreated patients, suggesting their ability to predict prognosis in patients who were deemed fit to receive chemotherapy as well as in those where only best supportive care was felt appropriate. Thus, the introduction of mGPS and NLR may promote an impartial clinical decision-making that will have a direct impact on patient care both in the active treatment and in the palliation setting. In particular, we have derived a model that combines two inflammation-based parameters that results in further prognostic accuracy. These scores are readily applicable to daily clinical practice using routine blood parameters.
Previous work has suggested that the patients with a diagnosis of CUP should be categorised into favourable and unfavourable groups prior to considering therapy. PS⩾2, hypoalbuminaemia, hypercalcaemia, raised LDH and number of metastatic sites have all been shown to be poor prognostic factors (Seve et al, 2006a,b; Trivanovic et al, 2009; Kodaira et al, 2010; Chen et al, 2012). Although our study has demonstrated a negative trend with some of these factors no statistical significant associations with OS were observed. Variations between our results and those previously published maybe explained by the renown heterogeneity of CUP which may explain the variability in assessing prognostic traits. There is limited published literature to suggest the role of tumour markers as a prognostic marker in CUP (Milovic et al, 2002). However, in future studies it may be of interest to further evaluate the interplay between tumour marker burden and inflammatory scores.
The relatively small sample size of the training set and the retrospective nature of our study should be acknowledged as potential limitations. In addition, although there is a postulated pathological inflammatory mechanism in carcinogenesis that forms the basis of both the NLR and mGPS, it must be recognised that infection and concomitant therapy with corticosteroids are also common causes for a measurable inflammatory response which may alter the prognostic utility of these parameters. Nonetheless, the number of deaths on which our survival analysis was powered, in conjunction with the process of external validation in a larger and independent case series leaves little doubt regarding the utility of mGPS and NLR as predictors of survival in patients with CUP.
Although the number of studies supporting the clinical use of inflammation-based prognostic models is increasing, including a study of 25 000 patients showing the stage and histotype-independence of the prognostic information produced by inflammatory scores in solid tumours, the molecular mechanisms sustaining cancer-related inflammation remains poorly understood (Ramsey et al, 2007; Sharma et al, 2008; Proctor et al, 2011). It has been postulated that tumour hypoxia and necrosis triggers a variety of host responses. These include an alteration in the metabolic, haematopoietic and neuroendocrine environment including inflammatory cytokines and haematopoietic growth factors. Numerous cytokines have been implicated in the pro-inflammatory response associated with malignancy but no one cytokine has been found to be pathogenic and it is likely that a complex interplay of cytokines drive the inflammatory process. For example, a recent study in patients with advanced colorectal cancer failed to show any correlation between NLR and circulating levels of IL-6 or TNF-α suggesting that further research is required in this area in order to define the possible cytokine relationship with carcinogenesis, especially as this may yield a therapeutic strategy to modulate the inflammatory response (Chua et al, 2012).
The NLR is associated with a derangement in CRP, corresponding to a pathological inflammatory state in cancer (Chua et al, 2012). A neutrophilic environment, which corresponds to a deranged NLR, results in infiltration of tumour cells and is responsible for the transformation to a metastatic phenotype (Tazawa et al, 2003). This maybe a result of protease-mediated extracellular digestion of the tumour basement membrane (Engbring and Kleinman, 2003). This degradation leads to the release of growth factors and other pro-mitotic cytokines. Thus, derangement of the NLR reflects a pro-inflammatory milieu which may contribute to a poor prognosis.
Hypoalbuminaemia is a recognised poor prognostic factor in CUP and represents a state of progressive malnutrition. The inflammatory response is a critical driver of cancer-related cachexia, where a hyper-catabolic state results in increased toxicity and reduced tolerance of chemotherapy (Esper and Harb, 2005).
In summary, the present study supports the prognostic utility of systemic inflammation-based scores in CUP. The mGPS and NLR are easily deducible, universally available and inexpensive prognosticators that can be routinely used in clinical practice and are both a more objective measure of patients’ prognosis when compared with PS. Thus, measurement of systemic inflammation should be considered in the routine assessment of patients with CUP.
Change history
07 January 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ando M, Ando Y, Hasegawa Y, Shimokata K, Minami H, Wakai K, Ohno Y, Sakai S (2001) Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer 85: 1634–1639.
Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D (2003) Performance status score: do patients and their oncologists agree? Br J Cancer 89: 1022–1027.
Chen KW, Liu CJ, Lu HJ, Tzeng CH, Liu JH, Chiou TJ, Yen CC, Wang WS, Chao TC, Teng HW, Chen MH, Liu CY, Chang PM, Yang MH (2012) Evaluation of prognostic factors and the role of chemotherapy in unfavorable carcinoma of unknown primary site: a 10-year cohort study. BMC Res Notes 5: 70.
Chua W, Clarke SJ, Charles KA (2012) Systemic inflammation and prediction of chemotherapy outcomes in patients receiving docetaxel for advanced cancer. Support Care Cancer 20: 1869–1874.
Crumley AB, Stuart RC, Mckernan M, Mcdonald AC, McMillan DC (2008) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol 23: e325–e329.
Engbring JA, Kleinman HK (2003) The basement membrane matrix in malignancy. J Pathol 200: 465–470.
Esper DH, Harb WA (2005) The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 20: 369–376.
Fearon KC, Voss AC, Hustead DS (2006) Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83: 1345–1350.
Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G (2011) Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22, (Suppl 6 vi64–vi68.
Forrest LM, McMillan DC, Mcardle CS, Angerson WJ, Dunlop DJ (2004) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 90: 1704–1706.
Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N (2009) Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev 35: 570–573.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88 (1): 218–230.
Harrell F (2010) RMS: REgression Medelling Strategies. R package version 3: 0–0.
Kodaira M, Takahashi S, Yamada S, Ueda K, Mishima Y, Takeuchi K, Yamamoto N, Ishikawa Y, Yokoyama M, Saotome T, Terui Y, Hatake K (2010) Bone metastasis and poor performance status are prognostic factors for survival of carcinoma of unknown primary site in patients treated with systematic chemotherapy. Ann Oncol 21: 1163–1167.
Mayordomo JI, Guerra JM, Guijarro C, Garcia-Prats MD, Gomez A, Lopez-Brea M, Gonzalez R, Hergueta P, Lopez-Pino MA, Martinez-Tello F et al (1993) Neoplasms of unknown primary site: a clinicopathological study of autopsied patients. Tumori 79: 321–324.
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67: 257–262.
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39: 534–540.
Milovic M, Popov I, Jelic S (2002) Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit 8: MT25–MT30.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, Mcfadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649–655.
Pavlidis N (2012) Optimal therapeutic management of patients with distinct clinicopathological cancer of unknown primary subsets. Ann Oncol 23 (Suppl 10): x282–x285.
Pavlidis N, Fizazi K (2009) Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 69: 271–278.
Pavlidis N, Petrakis D, Golfinopoulos V, Pentheroudakis G (2012) Long-term survivors among patients with cancer of unknown primary. Crit Rev Oncol Hematol 84: 85–92.
Penel N, Negrier S, Ray-Coquard I, Ferte C, Devos P, Hollebecque A, Sawyer MB, Adenis A, Seve P (2009) Development and validation of a bedside score to predict early death in cancer of unknown primary patients. PLoS One 4: e6483.
Petrakis D, Pentheroudakis G, Voulgaris E, Pavlidis N (2013) Prognostication in cancer of unknown primary (CUP): development of a prognostic algorithm in 311 cases and review of the literature. Cancer Treat Rev 39: 701–708.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'reilly DS, Foulis AK, Horgan PG, McMillan DC (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 47: 2633–2641.
Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC (2007) Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 109: 205–212.
Seve P, Ray-Coquard I, Trillet-Lenoir V, Sawyer M, Hanson J, Broussolle C, Negrier S, Dumontet C, Mackey JR (2006a) Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer 107: 2698–2705.
Seve P, Sawyer M, Hanson J, Broussolle C, Dumontet C, Mackey JR (2006b) The influence of comorbidities, age, and performance status on the prognosis and treatment of patients with metastatic carcinomas of unknown primary site: a population-based study. Cancer 106: 2058–2066.
Sharma R, Hook J, Kumar M, Gabra H (2008) Evaluation of an inflammation-based prognostic score in patients with advanced ovarian cancer. Eur J Cancer 44: 251–256.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P (2009) Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 197: 466–472.
Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M (2003) Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol 163: 2221–2232.
Trivanovic D, Petkovic M, Stimac D (2009) New prognostic index to predict survival in patients with cancer of unknown primary site with unfavourable prognosis. Clin Oncol (R Coll Radiol) 21: 43–48.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91: 181–184.
Acknowledgements
The authors would like to acknowledge Dr Les Huson for his statistical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Mohamed, Z., Pinato, D., Mauri, F. et al. Inflammation as a validated prognostic determinant in carcinoma of unknown primary site. Br J Cancer 110, 208–213 (2014). https://doi.org/10.1038/bjc.2013.683
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.683
Keywords
This article is cited by
-
Extensive diagnostic work-up for patients with carcinoma of unknown primary
Clinical & Experimental Metastasis (2021)
-
Integrating histopathology, immune biomarkers, and molecular subgroups in solid cancer: the next step in precision oncology
Virchows Archiv (2019)
-
2018 consensus statement by the Spanish Society of Pathology and the Spanish Society of Medical Oncology on the diagnosis and treatment of cancer of unknown primary
Clinical and Translational Oncology (2018)
-
Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer
British Journal of Cancer (2014)