Abstract
Background:
We aimed to determine the role of palliative resection in metastatic colorectal cancer (mCRC) and ascertain which patient populations would benefit most from this treatment.
Methods:
A total of 1015 patients diagnosed with mCRC at Seoul National University Hospital between 2000 and 2009 were retrospectively studied.
Results:
Of the 1015 patients, 168 patients with only liver and/or lung metastasis received curative resection. The remaining 847 patients were treated with palliative chemotherapy and/or palliative resection combined with best supportive care. Palliative resection was performed in 527 (62.2%) cases (complete resection with negative margin (R0) in 93, R1/2 in 434). Resected patients had a more prolonged median overall survival (OS) than unresected patients (21.3 vs 14.1 months; P<0.001). In multivariate analysis, R0 resection was found to be associated with a superior OS compared with R1/2 resection (51.3 vs 19.1 months; P<0.001) and no resection (51.3 vs 14.1 months; P<0.001). When we performed propensity score matching, palliative resection was found to be related to prolonged OS (hazard ratio=0.72, 95% confidence interval=0.59–0.89; P=0.003).
Conclusion:
Palliative resection without residual disease and chemotherapy confers a longer-term survival outcome than palliative chemotherapy alone in mCRC patient subset.
Similar content being viewed by others
Main
Approximately 20% of patients with colorectal cancer (CRC) have distant metastatic disease at the time of presentation (Jemal et al, 2009). The majority of patients with metastatic CRC (mCRC) cannot be cured, although a subset of these cases with liver- and/or lung-isolated disease is potentially curable with surgery (Ballantyne and Quin, 1993; Regnard et al, 1998; Headrick et al, 2001).
Systemic chemotherapy is the standard approach in treating mCRC and the last 5–10 years have seen unprecedented advances in therapies for this cancer. When 5-fluorouracil was the sole active agent, overall survival (OS) in mCRC cases was approximately 11–12 months. In recent years, however, the average median survival duration has doubled. This increase has been mainly driven by the availability of new active agents, such as irinotecan, oxaliplatin, cetuximab, panitumumab, and bevacizumab (Cunningham et al, 2004; Grothey et al, 2004; Hurwitz et al, 2004; Sobrero et al, 2008). Recently, the FDA has approved the angiogenesis inhibitor aflibercept for patients with previously treated mCRC. Although many new drugs are currently being investigated, they have not yet changed the standard treatment nor improved treatment outcomes to any significant extent.
In contrast, the role of palliative resection as an intervention for mCRC has been restricted. Resection of the primary site is performed in order to manage symptoms, such as obstruction, perforation, and bleeding, but the use of bowel resection in relatively asymptomatic patients with stage IV disease has not been well defined (Liu et al, 1997; Scoggins et al, 1999; Rosen et al, 2000). The rationale for immediate resection in asymptomatic patients is based on the prevention of primary-related complications later on during the treatment course, which can require urgent surgery and are associated with higher mortality. Furthermore, there are very few data on palliative metastasectomies confined to the peritoneum and ovary (Miller et al, 1997; Mahteme et al, 2004; Erroi et al, 2007). Previously, studies on these treatments were retrospective in nature and had small sample sizes with short-term follow-up periods, making it difficult to determine the benefits of such surgeries with any certainty.
In this retrospective study, we evaluated the treatment outcomes of palliative resection combined with standard chemotherapy in mCRC patient cohort. In addition, we attempted to identify clinical predictive factors that can determine the benefits of palliative resection.
Materials and Methods
Patients
A review of hospital databases was performed to identify patients with a diagnosis of stage IV mCRC between 2000 and 2009 (Figure 1). The number of patients who had histologically confirmed adenocarcinoma of the colon and rectum with distant metastasis was 1015. We divided our sample group into two groups according to the extent of metastasis as follows: (1) patients with resectable liver/lung metastasis who had curative potential; and (2) mCRC patients who were not candidates for curative surgery. Data on age, gender, tumour site, Eastern Cooperative Oncology Group (ECOG) performance status, primary tumour location, pathological differentiation, the organs involved by CRC, chemotherapy, and surgery were retrieved by reviewing patient medical records. The extent of tumour spread was determined from the pretreatment workup (chest X-ray, computed tomography, or positron emission tomography). Intraoperative findings documented in the operative report also contributed to the determination of metastatic tumour involvement.
The data are presented as frequencies and percentages for categorical variables and were analysed with the Pearson’s χ2 test or the Fisher’s exact test. The Student’s t-test and Mann–Whitney U-test were used for statistical comparison between the groups. The median duration of OS was calculated using the Kaplan–Meier method. Comparisons between different groups were made using the log-rank test. Multivariate analyses were performed using a logistic regression model for responses and a Cox regression model for OS to identify independent factors and adjust for baseline characteristics. Two-sided P-values of less than 0.05 were considered significant. All analyses were performed using SPSS for Windows, version 19.0 (IBM Corporation, Armonk, NY, USA).
Because of the nonrandomised, observational nature of this study, we performed propensity score matching to evaluate the efficacy of palliative resection. Briefly, the propensity to undergo palliative resection or not was scored using a multivariable logistic regression for each patient, using five variables that affected the OS. This study was approved by the Institutional Review Board of Seoul National University Hospital.
Results
Patient characteristics
Patient characteristics are listed in Table 1. The ages ranged from 16 to 88 years (median age, 61 years) and 595 patients (58.6%) were male. All tumours in this cohort were either adenocarcinoma or carcinoma. Primary tumours were located in the right colon (caecum, ascending, or transverse colon) in 393 patients (38.7%), descending and rectosigmoid colon in 63 patients (6.3%) and 549 patients (54.1%), respectively. The most common site of metastatic disease at presentation was the liver (589 patients, 58.0%), followed by distant lymph nodes (267 patients, 26.3%), peritoneum (251 patients, 24.7%), lung (203 patients, 20.0%), and ovary (48 patients, 4.7%). The median number of metastatic sites was 1 (range, 1–6) and 905 (89.2%) cases had limited metastasis (number of metastatic sites ⩽2). The median OS in the total study population was 21.9 months (95% confidence interval (CI)=20.4–23.4).
Treatment outcomes in mCRC patients who underwent curative metastasectomy of the lung and/or liver
Of the total cohort of 1015 patients under analysis, 168 (16.5%) received a lung or liver metastasectomy with curative intent. Curative resection of the liver and lung was done in 144 (85.7%) and 27 patients (16.1%), respectively. Liver and lung metastasectomies were simultaneously performed in three patients. The median OS was 82.5 months (95% CI=63.2–101.8) in these populations (Figure 2A). After resection, 158 patients (94.0%) received postoperative chemotherapy. In univariate analysis, a young age (<62; not reached vs 55.1 months; P=0.020) and chemotherapy after resection (82.5 vs 43.9 months; P=0.006) were found to be associated with prolonged OS. There was no interaction between the metastatic organ involved and median OS. In multivariate analysis, chemotherapy was the only significant predictor of good prognosis (P=0.030, hazard ratio (HR)=0.31, 95% CI=0.11–0.89).
Treatment outcomes in patients who were not candidates for curative metastasectomy/palliative resection
Palliative resection was done in 527 patients (62.2%), complete resection with negative margin (R0) in 93 (17.6%), complete resection with positive margin (R1) in 10 (1.9%), and grossly residual disease after resection (R2) in 424 (80.5%) cases. Of the 93 patients who underwent R0 resection, 51 (54.8%) with synchronous metastasis underwent resection of both the primary site and metastasis, whereas resection of only the metastatic site was performed in 42 (45.1%) patients with metachronous metastasis. Eighty patients (86.0%) had limited metastasis (number of metastatic organs ⩽2) involving the peritoneum (27 patients, 29%), local recurrence (21 patients, 24.7%), lymph nodes (14 patients, 15.1%), ovaries (14 patients, 15.1%), liver (11 patients, 11.8%), and lung (5 patients, 5.4%). The performance status of the majority of these patients was ECOG 0–1 (90 patients, 96.7%) and only 3 patients (3.2%) showed an ECOG 2 rating. The major reason for palliative resection was reduction of tumour burden in asymptomatic patients (347, 65.8%). The surgery for palliation of symptoms (obstruction, perforation, or bleeding) was performed in 112 (21.3%), 25 (4.7%), and 5 patients (0.9%), respectively. In 35 patients (6.6%), distant metastasis was detected via baseline computed tomography immediately after surgeries with curative intent.
Of the 527 patients in our cohort who received palliative resection, resection of both primary and metastatic sites was done in 108 cases (20.5%). Primary or metastatic sites alone were resected in 337 (63.9%) and 82 patients (15.6%), respectively.
Chemotherapy
Of 847 patients who were not candidates for treatment with curative intent, 88 patients (10.4%) did not receive chemotherapy either due to the patient’s refusal or a poor performance status and 13 patients (1.5%) insisted on a referreal to a local hospital. In 746 cases where a history of palliative chemotherapy was documented, a median two lines of chemotherapy were performed (range=1–9) and 361 patients (68.5%) received two or more lines of chemotherapy. Oxaliplatin was the most commonly used agent (685 patients, 80.9%) and irinotecan was administered in 570 patients (67.3%). 5-Fluorouracil and capecitabine were employed in 675 (79.7%) and 399 patients (47.1%), respectively. Finally, 103 patients (18.1%) received cetuximab, whereas bevacizumab was used in 90 (10.6%) cases.
There was a significant difference in the median progression-free survival associated with first-line chemotherapy between patients with palliative resection and no resection (7.7 vs 6.6 months; P<0.001). In particular, the median progression-free survival in patients who underwent R0 resection was much longer than in patients who underwent R1/2 resection and those who were not resected (13.6 vs 6.7 vs 6.6 months; P<0.001).
Survival outcomes according to clinical factors and treatment modalities
The median OS was 19.0 months (95% CI=17.8–20.1; Figure 2B) and the median OS in patients with palliative resection was longer than in patients with no resection (21.4 vs 14.1 months; P<0.001; Figure 3A). In particular, the median OS in patients with R0 resection was far superior to all other patients in the cohort (51.3 months; P<0.001; Figure 3B).
When we compared patients who received palliative resection with those who did not, there were differences in age and in the number of metastases. Patients who underwent palliative resection tended to be younger than those who did not receive this treatment (P=0.002). In addition, there was a noted trend that the proportion of patients who had a limited number of metastases (⩽2) was higher in the resection patient group, although this was not statistically significant (P=0.056). We also detected a tendency for the mean serum carcinoembryonic antigen (CEA) value to be higher in cases who did not undergo resection (P=0.056). We summarised these comparisons between patients with palliative resection and no resection in Table 2.
In univariate analysis, there were some good additional prognostic factors identified including an age <61 years, well-to-moderate differentiation, tumour location in the sigmoid colon and rectum, an ECOG 0–1, a lower than median CEA value (<9.3 ng ml−1), chemotherapy, number of metastatic sites ⩽2, and microsatellite stability or low microsatellite-instability. In multivariate analysis, R0 resection was found to be associated with prolonged OS compared with R1/2 resection and no resection. In addition, well-to-moderate differentiation, a good performance status (ECOG 0–1), a lower than median level of CEA, chemotherapy, and a low number of metastatic sites (number of metastatic sites ⩽2) remained clinically significant in multivariate analysis. Table 3 summarises the various prognostic factors for OS in CRC cases.
Figure 4 depicts the effects of palliative resection on survival for stratified mCRC patient subgroups. Palliative resection was found to be associated with significant risk reduction across all subgroups except for patients with a poorly differentiated histology and a low ECOG (⩾2). In addition, in patients ⩾71 years of age, palliative resection did not prolong OS significantly.
Propensity score matching
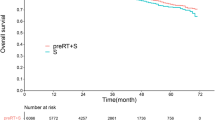
We used propensity score matching to compare the effects of palliative resection. In order to minimise the bias related to the nonrandom allocation of palliative resection, we developed a matching scheme that included variables that have been shown to be associated with OS in our present analyses. We included variables within the following domains: age, tumour differentiation, performance status, CEA level, number of metastases, and chemotherapy. We then used propensity score matching to match 213 patients (40.4% of the relevant group) who were treated with palliative resection to 213 patients (66.7% of the relevant group) who did not undergo this treatment. As shown in Figure 5, the Kaplan–Meier curves for the effects of palliative resection after matching on the propensity score showed a clear survival benefit associated with this intervention. Palliative resection reduced the hazard of death by 28% (HR=0.72, 95% CI=0.59–0.89) in a Cox regression model (P=0.003).
Discussion
In our current study, we analysed 1015 patients who were diagnosed with mCRC within a 10-year period. As is widely accepted, mCRC patients with only liver- and/or lung-isolated disease could achieve long-term survival through curative resection combined with systemic chemotherapy. In other mCRC patients, palliative resection followed by chemotherapy, especially if R0 resection is feasible, was found to be associated with a more prolonged OS than palliative chemotherapy alone.
Our study results indicate that palliative surgery is a valid treatment option in mCRC patients. With the exception of resectable liver/lung metastases, palliative resection in mCRC patients has been performed previously only to relieve symptoms or in emergent medical situations (perforation, obstruction, or bleeding) and clinicians generally choose systemic chemotherapy in asymptomatic mCRC patients. However, we demonstrate from our current analysis that mCRC patients, especially in those for whom R0 resection is achievable, had a significant survival benefit through surgical resection followed by systemic chemotherapy as compared with systemic treatment alone. Hence, aggressive surgical treatment combined with systemic chemotherapy should be considered in mCRC patients with a resectable metastasis because a portion of these patients has the possibility of achieving long-term disease-free survival and greatly improved OS.
When performing palliative resection, postoperative morbidity and mortality should be considered. For patients with mCRC who undergo surgery, there is a 20–30% risk of postoperative morbidity and a 1–6% risk of perioperative mortality (Scoggins et al, 1999; Ruo et al, 2003; Galizia et al, 2008). In our current study, the mortality rate at 1 month and 3 months after surgical resection was 0.9% (5 of 527 patients) and 4.9% (26 of 527 patients), respectively. The postoperative mortality rate measured in present analysis was thus similar to or lower than that reported previously reports and therefore at an acceptable level.
Clearly, only patients who would benefit from palliative resection should be considered for this procedure. As we noticed from our subgroup analysis, palliative resection did not significantly prolong the OS in patients with poorly differentiated tumours. Moreover, our subgroup analysis revealed that the benefit from palliative resection was not significant in patients ⩾71 years old or patients with a poor ECOG (⩾2). Based on these results, we recommend that palliative resection should be considered in younger patients (⩽70 years old) with a good performance status and not harbouring a poorly differentiated histology.
Previous data on the resection of metastatic sites have generally been confined to specific organs, such as the ovaries, retroperitoneal lymph nodes, and adrenal glands, and these were generated in small sample-sized studies (Rayson et al, 2000; McCormick et al, 2007; Mourra et al, 2008; Ho et al, 2011). In our current study, we analysed 527 patients who underwent palliative resection during a 10-year period and demonstrated the benefit of palliative resection regardless of metastatic site. Statistically, by matching resected and unresected patients using their propensity scores, we reduced the selection bias. This approach has been used successfully in a medical context in the past (Connors et al, 1996; Earle et al, 2001; Iwashyna and Lamont, 2002). Our present analyses were performed retrospectively and this is a major limitation of our current study. However, our cohort comprised consecutive patients who were diagnosed with mCRC to minimise selection bias.
In summary, surgery provides a potentially curative option for selected patients who present with non-liver/lung metastasis-related mCRC. If the metastases are potentially resectable and the performance status of the patients is good, resection of both the primary lesion and the metastasis may be a good treatment option. Before surgical resection, tumour differentiation and the patient’s age should be considered. With the integration of surgery and chemotherapy, a longer-term survival can be achieved than through palliative chemotherapy alone.
Change history
16 April 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ballantyne GH, Quin J (1993) Surgical treatment of liver metastases in patients with colorectal cancer. Cancer 71 (12 Suppl): 4252–4266
Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 276 (11): 889–897
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351 (4): 337–345
Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC (2001) Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol 19 (4): 1064–1070
Erroi F, Scarpa M, Angriman I, Cecchetto A, Pasetto L, Mollica E, Bettiol M, Ruffolo C, Polese L, Cillo U, D'Amico DF (2007) Ovarian metastasis from colorectal cancer: Prognostic value of radical oophorectomy. J Surg Oncol 96 (2): 113–117
Galizia G, Lieto E, Orditura M, Castellano P, Imperatore V, Pinto M, Zamboli A (2008) First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 143 (4): 352–358, discussion 358
Grothey A, Sargent D, Goldberg RM, Schmoll H-J (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22 (7): 1209–1214
Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, Pairolero PC (2001) Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 71 (3): 975–980
Ho TW, Mack LA, Temple WJ (2011) Operative salvage for retroperitoneal nodal recurrence in colorectal cancer: a systematic review. Ann Surg Oncol 18 (3): 697–703
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350 (23): 2335–2342
Iwashyna TJ, Lamont EB (2002) Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage iii colon cancer. J Clin Oncol 20 (19): 3992–3998
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer Statistics, 2009. CA Cancer J Clin 59 (4): 225–249
Liu SKM, Church JM, Lavery IC, Fazio VW (1997) Operation in patients with incurable colon cancer—Is it worthwhile? Dis Colon Rectum 40 (1): 11–14
Mahteme H, Hansson J, Berglund A, Pahlman L, Glimelius B, Nygren P, Graf W (2004) Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer 90 (2): 403–407
McCormick CC, Giuntoli RL, Gardner GJ, Schulick RD, Judson K, Ronnett BM, Vang R, Bristow RE (2007) The role of cytoreductive surgery for colon cancer metastatic to the ovary. Gynecol Oncol 105 (3): 791–795
Miller BE, Pittman B, Wan JY, Fleming M (1997) Colon cancer with metastasis to the ovary at time of initial diagnosis. Gynecol Oncol 66 (3): 368–371
Mourra N, Hoeffel C, Duvillard P, Guettier C, Flejou JF, Tiret E (2008) Adrenalectomy for clinically isolated metastasis from colorectal carcinoma: report of eight cases. Dis Colon Rectum 51 (12): 1846–1849
Rayson D, Bouttell E, Whiston F, Stitt L (2000) Outcome after ovarian/adnexal metastectomy in metastatic colorectal carcinoma. J Surg Oncol 75 (3): 186–192
Regnard J-F, Grunenwald D, Spaggiari L, Girard P, Elias D, Ducreux M, Baldeyrou P, Levasseur P (1998) Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg 66 (1): 214–218
Rosen SA, Buell JF, Yoshida A, Kazsuba S, Hurst R, Michelassi F, Millis JM, Posner MC (2000) Initial presentation with stage IV colorectal cancer: How aggressive should we be? Arch Surg 135 (5): 530–534
Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD (2003) Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 196 (5): 722–728
Scoggins C, Meszoely I, Blanke C, Beauchamp R, Leach S (1999) Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 6 (7): 651–657
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz H-J, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA (2008) EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26 (14): 2311–2319
Acknowledgements
This work was supported by the second phase of the Brain Korea 21 program in 2012 and Grant No. R31-2008-000-10103-0 from the WCU project of the MEST and NRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Park, J., Kim, TY., Lee, KH. et al. The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer 108, 1425–1431 (2013). https://doi.org/10.1038/bjc.2013.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.94
Keywords
This article is cited by
-
Individualized prediction of survival benefit from primary tumor resection for patients with unresectable metastatic colorectal cancer
World Journal of Surgical Oncology (2020)
-
A nomogram for predicting bowel obstruction in preoperative colorectal cancer patients with clinical characteristics
World Journal of Surgical Oncology (2019)
-
Pulmonary metastasis in newly diagnosed colon-rectal cancer: a population-based nomogram study
International Journal of Colorectal Disease (2019)
-
Benefit of Surgical Resection of the Primary Tumor in Patients Undergoing Chemotherapy for Stage IV Colorectal Cancer with Unresected Metastasis
Journal of Gastrointestinal Surgery (2018)
-
Adverse prognostic impact of the CpG island methylator phenotype in metastatic colorectal cancer
British Journal of Cancer (2016)