Abstract
Background:
Agents targeting programmed death-1 receptor (PD-1) and its ligand (PD-L1) are showing promising results in non-small-cell lung cancer (NSCLC). It is unknown whether PD-1/PD-L1 are differently expressed in oncogene-addicted NSCLC.
Methods:
We analysed a cohort of 125 NSCLC patients, including 56 EGFR mutated, 29 KRAS mutated, 10 ALK translocated and 30 EGFR/KRAS/ALK wild type. PD-L1 and PD-1 expression were assessed by immunohistochemistry. All cases with moderate or strong staining (2+/3+) in >5% of tumour cells were considered as positive.
Results:
PD-1 positive (+) was significantly associated with current smoking status (P=0.02) and with the presence of KRAS mutations (P=0.006), whereas PD-L1+ was significantly associated to adenocarcinoma histology (P=0.005) and with presence of EGFR mutations (P=0.001). In patients treated with EGFR tyrosine kinase inhibitors (N=95), sensitivity to gefitinib or erlotinib was higher in PD-L1+ vs PD-L1 negative in terms of the response rate (RR: P=0.01) time to progression (TTP: P<0.0001) and survival (OS: P=0.09), with no difference in PD1+ vs PD-1 negative. In the subset of 54 EGFR mutated patients, TTP was significantly longer in PD-L1+ than in PD-L1 negative (P=0.01).
Conclusions:
PD-1 and PD-L1 are differentially expressed in oncogene-addicted NSCLC supporting further investigation of specific checkpoint inhibitors in combination with targeted therapies.
Similar content being viewed by others
Main
Lung cancer is the leading cause of cancer-related mortality worldwide in men and is second only to breast cancer in women (Siegel et al, 2013). Non-small cell lung cancer (NSCLC) accounts for 75% of all lung cancers and includes different subtypes, underlying relevant biological differences. Most patients with NSCLC are diagnosed in advanced stage, with a 5-year survival rate of <5% (Goldstraw et al, 2007). During the past years, drugs targeting the epidermal growth factor receptor (EGFR), such as gefitinib, erlotinib or afatinib and drugs targeting anaplastic lymphoma kinase (ALK) translocation, such as crizotinib, are offering new hopes, respectively, to the patients with EGFR mutations or ALK translocations (Mok et al, 2009; Maemondo et al, 2010; Mitsudomi et al, 2010; Zhou et al, 2011; Han et al, 2012; Rosell et al, 2012; Sequist et al, 2013; Shaw et al, 2013; Wu et al, 2014). Nevertheless, even with targeted agents, no patient with metastatic NSCLC can obtain a definitive cure and inevitably disease progresses after a median of 8–12 months, highlighting the urgent need for additional and more effective strategies.
For many years, several investigators evaluated the possibility to modulate the immune system for treating lung cancer, with disappointing results (Topalian et al, 2011; Hall et al, 2013). Lack of efficacy was probably related to the ability of the tumour to activate several distinct pathways to escape immune activity, including endogenous ‘immune checkpoints’. Immunotherapy of cancer has entered into a new phase from the discovery of drugs able to interfere with specific immune checkpoints such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and, more recently, programmed death-1 receptor (PD-1) and its ligand (PD-L1).
Programmed cell death protein 1, also known as PD-1, is a 288 amino acid cell surface protein molecule. PD-1 has two ligands, PD-L1 and PD-L2, which are members of the B7 family. Several lines of evidence suggest that PD-1 and its ligands negatively regulate immune responses. Different studies have showed that cancer microenvironment manipulates the PD-L1/PD-1 pathway and that the induction of PD-L1 expression on tumour cells leads to the inhibition of immune responses against cancer, permitting cancer progression and metastasis (Drake et al, 2006; Pardoll, 2012). Spranger et al, 2013 demonstrated in a murine mechanistic experiments that the induction of PD-L1 in melanoma microenvironment is mediated by IFN-y produced by the T cells that are antigen experienced and therefore express PD-1.
Expression of PD-L1 has been correlated with poor clinical outcomes in a number of human cancers, including melanoma, lung, breast, bladder, ovarian, pancreatic cancers, oesophagus adenocarcinoma, kidney tumours as well as in hematopoietic malignancies (Zou and Chen, 2008). However, other reports indicated a lack of association between PD-L1 expression and outcome (Konishi et al, 2004; Mischinger et al, 2010) or that PD-L1 expression was associated with an improved survival and increased TILs (Taube et al, 2012).
Recent studies showed that, in tumour cells, PD-L1 expression may range from 45 to 50% in NSCLC biopsies, irrespective of histology (Grosso et al, 2013). The evidence that PD-L1 is commonly up-regulated in NSCLC and that PD-1 is expressed on the majority of tumour infiltrating lymphocytes, represented the rationale for the development of monoclonal antibodies against PD-L1 or PD-1, and several agents are currently under investigation. Preliminary results suggested that PD-L1 positivity may correlate with response to treatment with PD-1 pathway inhibitors (Topalian et al, 2012; Grosso et al, 2013). Moreover, recent data suggested that checkpoint inhibitors could be more effective in smokers (Soria et al, 2013), where somatic gene mutations are frequent, suggesting a potential different expression of PD-1/PD-L1 in presence of specific molecular events including KRAS mutations. In addition, a recent study demonstrated that expression of mutant EGFR in bronchial epithelial cells induced PD-L1, and PD-L1 expression was reduced by EGFR inhibitors in NSCLC cell lines with activated EGFR (Akbay et al, 2013).
Based on these premises we supposed that PD-1/PD-L1 expression could differ according to the molecular phenotype of the tumour. The aim of the present study was to assess whether PD-1/PD-L1 expression was differently expressed in NSCLC patients according to presence or absence of EGFR mutations, ALK translocations or KRAS mutations.
Materials and methods
Patient selection
This retrospective study was conducted in a cohort of 125 metastatic NSCLC patients followed in three Italian centres. We selected two cohorts of patients (EGFR mutated and EGFR wild type) with availability of additional tumour tissue from the same tumour sample previously used for EGFR assessment. In addition, we included onto the study only cases evaluated for KRAS and ALK status, with full clinical data including previous therapies and survival. EGFR mutations and KRAS mutations were evaluated using Polymerase Chain Reaction and direct sequencing, while presence of ALK translocations were detected using fluorescence in situ hybridisation. All tests were performed locally as a part of clinical practice. The study was approved by the local Ethics Committee and was conducted in accordance with the ethical principles stated in the most recent version of the Declaration of Helsinki or the applicable guidelines on good clinical practice, whichever represented the greater protection of the individuals.
Immunohistochemistry
Four-micron sections of 125 primary or metastatic NSCLC samples were used throughout this study. Standard indirect immunoperoxidase procedures were used for immunohistochemistry (IHC; ABC-Elite, Vector Laboratories, Burlingame, CA, USA). Briefly, slides were dewaxed and rehydrated in distilled water. Endogenous peroxidase activity was blocked using 0.5% H2O2. The sections were treated with 10% normal goat serum (DakoCytomation; Dako, Carpinteria, CA, USA) for 20 min and incubated with primary antibodies PD-L1 (CD274) ab58810 (Abcam, Cambridge, UK) (Bloch et al, 2013; Shi et al, 2013) and PD-1 760–4448 (Ventana, Tucson, AZ, USA) at room temperature. Sections were further incubated with peroxidase-labelled secondary antibody (DakoCytomation) for 30 min at room temperature. For visualisation of the antigen, the sections were immersed in 3-amino-9-ethylcarbazole plus substrate-chromogen (DakoCytomation) for 30 min, and counterstained with Gill’s hematoxylin.
Two well-experienced pathologists (MA and LT) examined the immunohistochemical slides without any prior information on the clinicopathological features of the patient samples.
Percentages of PD-L1 and PD-1 positive tumour cells and staining intensity were evaluated for each sample. Staining intensity was scored considering 0 as negative or trace, 1 as weak, 2 as moderate and 3 as high. In absence of any standardised scoring system, all cases with staining intensity ⩾2 in more than 5% of tumour cells were considered as positive, similarly to previous studies (Antonia et al, 2013; Grosso et al, 2013; Soria et al, 2013). Moreover, a semi-quantitative approach was used to generate a score for each tissue core. The percentage of stained cells (0–100%) was multiplied by the dominant intensity pattern of staining ranging from 0 to 3. Therefore, the overall semiquantitative score ranged from 0 to 300.
Statistical analyses
Sample size was computed assuming a difference of 25% in PD-1 or PD-L1 expression in EGFR mutated vs EGFR wild type. With a power of 80% and a significance level of 0.05 (1-tailed test), a sample size of at least 49 patients was required for each group. Statistical analyses were performed to compare differences between patients with and without PD-1 and PD-L1 expression according to presence or absence of a specific biomarker. Clinical characteristics and associations with biomarkers were examined comparing the differences by χ2-test or Fisher’s exact test as appropriate. Comparison between the two groups with or without PD-1 or PD-L1 expression was performed by log rank test. A multivariable analysis was performed using a logistic regression model in order to explore the association of PD-1/PD-L1 expression with patient characteristics (sex, smoke, histology, EGFR, KRAS and ALK). A step-down procedure method was selected. The criterion for variable removal was the likelihood ratio statistic based on the maximum partial likelihood estimates (default P-value of 0.10 for removal from the model). Differences between median score was performed by U-Mann–Witney test. Correlative analyses of PD-1 or PD-L1 expression and outcome of patients according to a specific therapy were evaluated. Time to progression (TTP) was calculated from date of therapy start to progression or last follow-up date; overall survival (OS) was calculated from the date of therapy start to death or last follow-up date, with 95% confidence intervals calculated using the Kaplan–Meier method (Kaplan and Meier, 1985). The significance level for all analyses was set at P<0.05 and all P-values were two-sided. Statistical analysis was performed by using IBM-SPSS Statistics version 20 (Property of IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 125 metastatic NSCLC patients were included in the study. A total of 98 (78.4%) samples were obtained from the primary tumour and 17 (13.6%) from the metastatic sites. This study included 99 cases treated with EGFR-TKIs (gefitinib: N=30, 30.3%; erlotinib: N=69, 69.7%) in first (N=29, 29.3%) or subsequent lines of treatment (N=70, 70.7%). The median age was 64 years (range: 41–84 years). The majority of patients were male (N=67, 53.6%), former (N=58, 46.4%) or current smokers (N=17, 13.6%) and mostly presented two metastatic sites (N=43, 34.4%). The most frequent metastatic sites were lung (N=72, 57.6%), followed by lymph-nodes (N=55, 44.0%), bone (N=37, 29.6%), brain (N=18, 14.4%), liver (N=13, 10.4%) and adrenal glands (N=8, 6.4%). Adenocarcinoma was the most frequent histology (N=83, 66.4%). All patients were analysed for presence of EGFR and KRAS mutation and for ALK translocation: this analysis included 56 (44.8%) EGFR mutated, 29 (23.2%) KRAS mutated, 10 (8.0%) ALK translocated and 30 (24.0%) EGFR/KRAS/ALK wild type, defined as triple negative. Exon 19 deletion (N=30, 24.0%) and codon 12 mutation (N=26, 20.8%), were the most frequent EGFR and KRAS alterations, respectively (Table 1). In this study, because of the criteria for patient selection, incidence of EGFR mutations, KRAS mutations and ALK translocations was not representative of a standard Caucasian population.
PD-1/PD-L1 expression and patient characteristics
PD-1 was successfully evaluated in 122 specimens. Median PD-1 expression was 30. As illustrated in Figure 1A–F, median PD-1 expression resulted high in male, in current smokers, in adenocarcinoma histology, in EGFR wild type, in ALK negative and in patients harbouring KRAS mutations. A total of 43 cases (35.2%) had moderate (2+) or strong (3+) staining in at least 5% of cells and were considered as PD1+ as illustrated in Figure 2 and in Supplementary Figure S1. As reported in Table 2, PD-1 positive (+) patients were more frequently male with adenocarcinoma histology, even if the association was not statistically significant. PD-1 positivity was significantly associated with current smoking status (P=0.02) and with presence of KRAS mutations (P=0.006), while no significant association was observed with presence of EGFR mutations or ALK translocations. A multivariable analysis confirmed the significant association between PD-1 and KRAS mutations (P=0.05) (Supplementary Table S1).
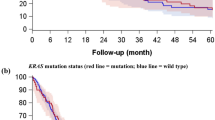
Levels of PD-1 expression and patient characteristics. This picture illustrates changes in the levels of PD-1 expression according to clinical (A–C) and biological (D–F) characteristics. Median levels of PD-1 score and interquartile ranges are showed. Median levels of PD-1 expression were higher in male (median score 60 vs 20) than in female (A), in current (median score 60 vs 20) than in never/former smokers (B), in adenocarcinoma (median score 40 vs 0) than in squamous-cell carcinoma histology (C), in EGFR wild type (median score 40 vs 20) than in EGFR mutated (D), in KRAS mutated (median score 60 vs 25) than in KRAS wild type (E) and in ALK wild type (median score 35 vs 15) than in ALK translocated (F) patients.
PD-1 and PD-L1 immunohistochemistry analysis. This figure illustrates four cases of PD-1 IHC analysis (A–D) and four cases of PD-L1 IHC analysis (E–H). Specifically, this picture showed: a PD-1 negative case (A), a PD-1 1+ case in 60% of tumour cells (B), a PD-1 2+ case in 80% of tumour cells (C), a PD-1 3+ case in 95% of tumour cells (D), a PD-L1 negative case (E), a PD-L1 1+ case in 10% of tumour cells (F), a PD-L1 2+ case in 50% of tumour cells (G) and a PD-L1 3+ case in 70% of tumour cells (H). The magnification used for the images was 20 × .
PD-L1 was successfully evaluated in 123 specimens, with a median expression level of 75. As illustrated in Figure 3A–F, median PD-L1 expression was high in female, in never/former smokers, in adenocarcinoma histology, in patients harbouring EGFR mutations, and in patients with ALK translocations. A score of 2+ or 3+ in >5% of tumour cells (PD-L1+) was observed in 68 cases (55.3%), as illustrated in Figure 2. As reported in Table 2, PD-L1+ status was significantly associated to adenocarcinoma histology (P=0.005) and more frequently observed in female and in never/former smokers, even if the association was not statistically significant. Importantly, presence of EGFR mutations was significantly associated with PD-L1 positive status (P=0.001). Multivariable analysis confirmed the significant association between PD-L1 and EGFR mutations (P=0.002) and between PD-L1 and adenocarcinoma histology (P=0.10) (Supplementary Table S1).
Levels of PD-L1 expression and patient characteristics. This picture illustrates changes in the levels of PD-L1 expression according to clinical (A–C) and biological (D–F) characteristics. Median levels of PD-1 score and interquartile ranges are showed. Median levels of PD-L1 expression were higher in female (median score 80 vs 60) than in male (A), in never/former (median score 60 vs 30) than in current smokers (B), in adenocarcinoma (median score 100 vs 0) than in squamous-cell carcinoma histology (C), in EGFR mutated (median score 120 vs 20) than in EGFR wild type (D), in KRAS wild type (median score 80 vs 55) than in KRAS mutated (E) and in ALK translocated (median score 115 vs 70) than in ALK wild type (F).
A formal comparison between primary tumours and metastasis was limited by the low number of samples (only 17 cases from metastatic sites). To clarify whether our results could be influenced by the simultaneous presence of primary and metastatic tumour samples, we reanalyzed the data by excluding all metastatic cases. In this analysis we confirmed that PD-1 positivity was significantly associated with current smoking status (P=0.05) and with presence of KRAS mutations (P=0.02) and that PD-L1 positivity status was significantly associated with adenocarcinoma histology (P=0.007) and with presence of EGFR mutations (P<0.001) (Supplementary Table S2).
PD1/PD-L1 expression was evaluable in 29 triple negative cases. In such subgroup, median PD-1 expression was 40, a result that was similar to the median PD-1 expression observed in EGFR mutants (median 20), KRAS mutants (median 60) or ALK translocated (median 15), as reported in Table 2. In the same subgroup of triple negative patients, median PD-L1 was 20, lower than the median PD-L1 levels observed in EGFR mutants (median 120) or in ALK translocated (median 115) and similar to KRAS mutants (median 55). As reported in Table 2, when compared with KRAS mutants, triple negative patients were more frequently PD-1 negative (P=0.01). When compared with patients with EGFR mutations, or with KRAS mutations or with ALK translocations, triple negative individuals were more frequently PD-L1 negative, with a P-value of <0.001, 0.02 and 0.06, respectively.
PD-1/PD-L1 expression and outcome of patients treated with EGFR-TKIs
Because of the strong association of PD-1/PD-L1 expression and KRAS or EGFR mutations, we further investigated the potential effect of such biomarkers on sensitivity to anti-EGFR agents. With such purpose we analysed the outcome of 99 patients treated with gefitinib or erlotinib. EGFR-TKIs were given as front-line therapy in 29 patients including 26 EGFR mutated patients, and in second or subsequent lines of therapy in 70 individuals, including 29 EGFR mutated, 16 KRAS mutated and 25 triple negative. EGFR-TKIs were offered in the second or further lines of treatment in patients with KRAS mutations or in triple negative according to clinical practice (Kim et al, 2008; Shepherd et al, 2009; Cappuzzo et al, 2010). In the group of patients treated with erlotinib or gefitinib response rate (RR) was 47.5%, median TTP 7.9 months and median OS 18.0 months.
PD-1 was assessed in 96 specimens and a positive expression was observed in 28 (29.2%) cases. Among the 93 patients evaluable for response, no differences in terms of RR (46.2 vs 50.7%, P=0.70), TTP (7.0 months vs 8.0 months, P=0.97) and OS (17.8 months vs 18.9 months, P=0.82) were identified in PD-1 positive (N=26, 28.0%) vs PD-1 negative (N=67, 72.0%) cases, as illustrated in Figure 4A and B.
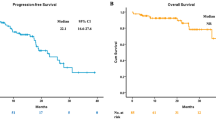
Kaplan–Meier curves of time to progression (TTP) ( A – C ) and overall survival (OS) ( B – D ) in patients treated with EGFR-TKIs. In the subgroup of patients (N=99) treated with EGFR-TKIs no differences in terms of TTP (7.0 months vs 8.0 months, P=0.97) (A) and OS (17.8 months vs 18.9 months, P=0.82) (B) were identified in PD-1 positive (N=26, 28.0%) vs PD-1 negative (N=67, 72.0%) cases. PD-L1 positive (N=49, 51.6%) patients had a significant longer TTP (11.7 months vs 5.7 months, P<0.0001) (C) and a longer OS (21.9 months vs 12.5 months, P=0.09) (D) than PD-L1 negative (N=46, 48.4%) patients.
PD-L1 was successfully evaluated in 98 specimens and PD-L1 positive expression was observed in 52 (53.1%) cases. Among the 95 patients treated with gefitinib or erlotinib and evaluable for response, PD-L1 positive (N=49, 51.6%) patients had significantly higher RR (61.2% vs 34.8%, P=0.01), significant longer TTP (11.7 months vs 5.7 months, P<0.0001) and longer OS (21.9 months vs 12.5 months, P=0.09) than PD-L1 negative (N=46, 48.4%) patients, as illustrated in Figure 4C and D.
In order to explore whether higher sensitivity to PD-L1 positive patients was related only to the concomitant presence of EGFR mutations or whether PD-L1 positive patients had a more indolent disease outcome when treated with EGFR-TKIs in presence of EGFR mutations, we further analysed the outcome of 54 EGFR mutated patients treated with gefitinib or erlotinib and evaluable for response. In this subgroup, although no difference in RR was detected (76.3% vs 75.0%, P=1.00), PD-L1 positive (N=38, 70.4%) patients had a significantly longer TTP (13.1 months vs 8.5 months, P=0.01) and a non significantly longer OS (29.5 months vs 21.1 months, P=0.75) than PD-L1 negative (N=16, 29.6%) patients, as reported in Figure 5A and B.
Kaplan–Meier curves of time to progression (TTP) ( A ) and overall survival (OS) ( B ) in PD-L1 positive vs PD-L1 negative EGFR mutated patients treated with EGFR-TKIs. In EGFR mutated patients (N=54) treated with EGFR-TKIs, PD-L1 positive (N=38, 70.4%) cases had a significantly longer TTP (13.1 months vs 8.5 months, P=0.01) (A) and a non-significantly longer OS (29.5 months vs 21.1 months, P=0.75) (B) than PD-L1 negative (N=16, 29.6%) cases.
Discussion
In the present study, the first specifically conducted in a cohort of molecularly selected NSCLC, we showed that expression of immune checkpoints differs according to tumour biology and patient characteristics. Patients harbouring KRAS mutations had higher levels of PD-1 expression when compared to the KRAS wild-type population and presence of EGFR mutations or ALK translocations were associated with increased PD-L1 protein levels. Moreover, the clinical profile of patients expressing PD-1 was different from the profile of individuals expressing PD-L1. Patients expressing PD-1 were more frequently male, smokers with adenocarcinoma, whereas patients expressing PD-L1 were more frequently female, never/former smokers with adenocarcinoma, the typical clinical features of KRAS mutants or EGFR mutants, respectively.
During the past few years, a growing interest surrounded immunotherapy and several agents targeting molecules involved in regulation of the immune system are under development. Agents targeting PD-1 and PD-L1 showed promising results in patients with NSCLC, particularly in individuals with high PD-L1 expression. PD-L1 is frequently found highly expressed in many human cancer types including NSCLC, being up-regulated in tumours by activation of key oncogenic pathways, such as the PI3KCA-AKT and the RAS-RAF-MAPK pathways (Parsa et al, 2007). These findings suggested that high mutational rates may contribute to increased immunogenicity (Chen et al, 2012) indirectly predicting sensitivity to checkpoint inhibitors (Soria et al, 2013). In a recent study, Antonia et al, 2013 showed that PD-L1 levels were numerically higher in individuals with EGFR mutations. Nevertheless, the very low number of patients with EGFR mutations included in this study, only four cases, precluded any firm conclusion.
Importantly, we described for the first time PD-1 expression on tumour cells. This finding could have therapeutic implications and need additional confirmation. The lack of additional tumour tissue precluded the possibility to perform further experiments.
Interestingly, it seems that mechanisms of immune system deregulation differ according to the type of mutation, with potential therapeutic implications. PD-1 expression was significantly associated with presence of KRAS mutations, while PD-L1 was strongly associated with presence of EGFR mutations, potentially modulating sensitivity to anti-EGFR agents. In a recent study, Akbay et al, 2013, showed that activation of the EGFR pathway induces PD-L1 expression to facilitate evasion of the host anti-tumour immune response. This role of EGFR signalling was independent of its effects on cell proliferation and survival, suggesting an active role for the EGFR oncogene in remodelling the immune microenvironment. Moreover, pharmacological blockade of the PD-1 pathway using EGFR-TKIs reduced PD-L1 expression, leading to tumour reduction, with a positive impact on overall survival (Akbay et al, 2013). The same effect was observed in our study. Patients harbouring EGFR mutations with high PD-L1 expression resulted more sensitive to gefitinib or erlotinib probably because of PD-L1 downregulation induced by the EGFR inhibition. The evidence that PD-L1 expression is a potential negative prognostic factor in several cancers further supports the hypothesis that the observed improvement in TTP was related to an indirect effect of the therapy on checkpoint expression (Zou and Chen, 2008). Overall, our and other data suggest that combination of PD-1 blockade with EGFR TKIs may be a promising therapeutic strategy to extend the duration of treatment response and delay development of resistance (Akbay et al, 2013).
Another interesting finding was the evidence that levels of PD-L1 were higher in patients with ALK translocations. Although the association was not statistically significant and although we were not able to evaluate whether a different checkpoint expression impacted on efficacy of anti-ALK agents, median PD-L1 levels were 5 times higher in ALK positive when compared with triple negative patients. Similarly to EGFR mutations, a potential effect of ALK translocation on checkpoint expression cannot be excluded and additional studies are recommended in this particular NSCLC subpopulation.
In the present study, we adopted two different methods for PD-1 or PD-L1 expression analyses, including a semiquantitative method largely adopted by our group in previous studies with biomarkers in NSCLC (Cappuzzo et al, 2005a, 2005b). Although the usage of a new scoring system could represent a potential limitation, such method allowed us to assess more properly the association of checkpoint expression with patient characteristics.
In conclusion, our study showed that immune checkpoints are differently expressed in oncogene-addicted NSCLC potentially modulating sensitivity to targeted agents. Our findings represent the rationale to choose a different checkpoint inhibitor according to the tumour driver and to combine targeted therapies with anti-PD-L1 or anti-PD-1 agents. Considering the retrospective nature of our investigation, additional prospective studies are warranted.
Change history
06 January 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3: 1355–1363.
Antonia S, Grosso J, Horak C, Harbison C, Kurland J, Inzunza D, Gupta A, Sankar V, Park JS, Jure-Kunkel M, Novotny J, Cogswell J, Zhang X, Phillips T, Simmons P, Simon J (2013) Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol 8 (suppl): abstract P2.11-035.
Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT (2013) Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 19: 3165–3175.
Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L, Franklin WA, Crino L, Gazdar AF, Bunn Jr PA, Hirsch FR (2005a) Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol 23: 5007–5018.
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn Jr PA, Varella-Garcia M (2005b) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 97: 643–655.
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W, Melezínek I, Klingelschmitt G, Klughammer B, Giaccone G SATURN investigators (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521–529.
Chen DS, Irving BA, Hodi FS (2012) Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18: 6580–6587.
Drake CG, Jaffee E, Pardoll DM (2006) Mechanisms of immune evasion by tumors. Adv Immunol 90: 51–81.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2: 706–714.
Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, Sankar V, Park JS, Kollia G, Taube JM, Anders R, Jure-Kunkel M, Novotny J, Taylor CR, Zhang X, Phillips T, Simmons P, Cogswell J (2013) Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 31 (suppl): abstract 3016.
Hall RD, Gray JE, Chiappori AA (2013) Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control 20: 22–31.
Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, Lee JS, Yoon SJ, Han JH, Lee JW, Jo SJ, Lee JS (2012) First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30: 1122–1128.
Kaplan EL, Meier P (1985) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372: 1809–1818.
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Aita H, Nishimura M (2004) B7-H1 expression on non small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10: 5094–5100.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388.
Mischinger J, Froehlich E, Griesbacher A, Pummer K, Mannweiler S, Zigeuner R, Lipsky K (2010) Prognostic relevance of B7H1 and B7H3 protein expressions in metastatic clear cell renal cell carcinoma. J Clin Oncol 28 (suppl): abstr e150674.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957.
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264.
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13: 84–88.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246.
Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327–3334.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O'Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA (2013) Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368: 2385–2394.
Shepherd FA, Douillard J, Fukuoka M, Saijo N, Kim S, Cufer T, Sellers MV, Armour AA, Kim ES (2009) Comparison of gefitinib and docetaxel in patients with pretreated advanced non-small cell lung cancer (NSCLC): Meta-analysis from four clinical trials. J Clin Oncol 27 (suppl): abstr 8011.
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH (2013) B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One 8: e76012.
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30.
Soria JC, Cruz C, Bahleda R, Delord JP, Horn L, Herbst RS, Spigel D, Mokatrin A, Fine G, Gettinger S (2013) Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). Eur J Cancer 49 (suppl): abstract 3408.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF (2013) Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5: 200ra116.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4: 127ra37.
Topalian SL, Weiner GJ, Pardoll DM (2011) Cancer immunotherapy comes of age. J Clin Oncol 29: 4828–4836.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454.
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15: 213–222.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742.
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumor microenvironment. Nat Rev Immunol 8: 467–477.
Acknowledgements
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG 2012-13157), Fondazione Ricerca Traslazionale (FoRT) and Istituto Toscano Tumori (ITT) (Project F13/16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
D'Incecco, A., Andreozzi, M., Ludovini, V. et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 112, 95–102 (2015). https://doi.org/10.1038/bjc.2014.555
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.555
Keywords
This article is cited by
-
PD-L1 expression and its significance in advanced NSCLC: real-world experience from a tertiary care center
Journal of the Egyptian National Cancer Institute (2024)
-
Overall survival in patients with advanced non-small cell lung cancer with KRAS G12C mutation with or without STK11 and/or KEAP1 mutations in a real-world setting
BMC Cancer (2023)
-
Clinical benefit of pembrolizumab in treatment of first line non-small cell lung cancer: a systematic review and meta-analysis of clinical characteristics
BMC Cancer (2023)
-
Correlation of PD-1 and PD-L1 expression in oral leukoplakia and oral squamous cell carcinoma: an immunohistochemical study
Scientific Reports (2023)
-
Effect of BIM expression on the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs
Scientific Reports (2023)