Abstract
Background:
Although use of menopausal hormone therapy (MHT) and some reproductive factors have been associated with colorectal cancer (CRC) risk, relations between these factors and survival after CRC diagnosis are unclear.
Methods:
Among 2053 post-menopausal women diagnosed with incident CRC in the NIH-AARP Diet and Health Study, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs) using multivariable Cox proportional hazards regression to test associations between oral contraceptive (OC) use, menarche age, age at first birth, parity, menopausal age, and MHT use with all-cause and CRC-specific mortality.
Results:
There were 759 deaths (332 CRC-related deaths) over a median follow-up of 7.7 years. We observed no statistically significant associations between OC use, menarche age, age at first birth, parity, menopausal age, and mortality. Compared with never MHT use, former use was not associated with mortality, but we found an inverse association among baseline current users, for both all-cause (HR=0.79, 95% CI 0.66–0.94) and CRC mortality (0.76, 0.59–0.99).
Conclusion:
Future studies should further focus on the mechanisms by which exogenous oestrogen exposure might affect tumour progression and CRC survival.
Similar content being viewed by others
Main
There were an estimated 624 340 female colorectal cancer (CRC) survivors in the United States in 2014 (American Cancer Society, 2014). This number is projected to grow to over 771 000 by 2024, making female survivors of CRC the second largest group of female cancer survivors in the United States (American Cancer Society, 2014). CRC rates are lower among women in the United States than among men (Brenner et al, 2007), and use of menopausal hormone therapy (MHT) has been associated with a 30–40% lower risk of CRC, prompting research into the role of oestrogen in carcinogenesis (Grodstein et al, 1999).
In addition to the well-established association between MHT use and lower CRC risk (Writing Group for the Women's Health Initiative I, 2002), previous literature has explored the hypotheses of exogenous oestrogen exposure and better survival after a CRC diagnosis (Persson et al, 1996; Slattery et al, 1999; Mandelson et al, 2003; Chan et al, 2006; Ritenbaugh et al, 2008; Newcomb et al, 2009). However, published results are conflicting, perhaps owing to small sample sizes and limited information on type and duration of MHT use. Also, while some studies, including an analysis in the NIH-AARP cohort (Zervoudakis et al, 2011), have suggested associations between reproductive factors (e.g., older age at menopause and older age at birth of first child) and higher CRC risk, studies on such reproductive factors and CRC survival are limited (Jacobsen et al, 1995).
Understanding factors that contribute to CRC survival may be useful to clinicians monitoring survivors and may influence research on disease progression. In this large, prospective cohort, we hypothesised that we would confirm previous findings on MHT use and improved survival; furthermore, we hypothesised that we would observe an inverse association between reproductive factors that increase oestrogen exposure and mortality risk among women with CRC.
Materials and Methods
Study population
The NIH-AARP Diet and Health Study has been previously described (Schatzkin et al, 2001). Briefly, the NIH-AARP cohort included 566,398 AARP members (aged 50–71 years) who completed a mailed baseline questionnaire in 1995–1996. An additional risk factor questionnaire was sent out 6 months after baseline with more detailed questions on MHT type and duration of use. Participants resided in one of six US states or two metropolitan areas. After excluding men, questionnaires completed by proxy, and individuals with a cancer diagnosis before baseline or end-stage renal disease, the cohort consisted of 365 255 women who were followed for incident CRC.
Cancer cases were identified by linking cohort members to eight original state cancer registries and three additional states through 31 December 2006. Cancer registries provided information on cancer diagnosis date, histology, stage, grade, and first course of treatment reported within 1 year of diagnosis. We classified invasive, CRC cases using histology codes from the International Classification of Diseases for Oncology, third edition (ICD-O-3 code C180–189, C199, and C209; Fritz, 2000). Incident cancer identification was estimated to be 90% complete (Michaud et al, 2005).
After excluding pre-menopausal women (n=54) and women diagnosed with in situ or metastatic CRC (n=417), 2053 invasive CRCs (resulting in 759 deaths overall, 332 CRC deaths) were included for analysis. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute.
Mortality ascertainment
Vital status was ascertained annually by linking the Social Security Administration Death Master File and the National Death Index Plus through 31 December 2011. We used ICD-9 and ICD-10 codes to classify deaths due to colon cancer (ICD-9 153 and 159.0, and ICD-10 C18–C26.0) and rectal cancer (ICD-9 154.0 and 154.1, and ICD-10 C19–C20). Accuracy of vital status ascertainment in this cohort is >95% (Hermansen et al, 2009).
Exposure assessment
The baseline questionnaire queried on various reproductive and hormonal factors, which we categorised as follows: age at menopause (<45, 45–49, 50–54, and 55+ years), age at first live birth (no births, <20, 20–29, and 30+ years), age at menarche (⩽12 and 13+ years), parity (nulliparous, 1–2 and 3+ children), oral contraceptive (OC) use (never/ever), MHT use (never, former, and current), and years using MHT (<5 and 5+). Additional analyses with more detailed categorisations for age at menarche and duration of OC use did not change the study conclusions; thus, we present categories as indicated above in the main analyses. Among the women in our sample who completed the risk factor questionnaire, we had additional information on hormone type (oestrogen only, progestin only, and oestrogen plus progestin) and duration for 1245 women.
Statistical analysis
We used IVEware 2.0 (Survey Methodology Program, Ann Arbor, MI, USA, 2002) to impute values for missing variables, using 10 iterations and five imputations. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards regression with age as the underlying time metric. Person-time was calculated from age at diagnosis to death or censoring. Proc mianalyze was used to combine results from the five imputed data sets.
In regression models, we included a priori determined covariates that were associated with the main exposures or mortality in previous analyses. Final models were adjusted for years from questionnaire to diagnosis (continuous), body mass index (18.5–25, 25–30, and 30+ kg m−2), marital status (married or living as married, yes/no), smoking status (never, former, and current), diabetes (yes/no), physical activity (never/rarely, 1–3 times per month, 1–2 times per week, 3–4 times per week, and 5+ times per week), tumour stage (localised and regional/distant), tumour grade (well differentiated, moderately differentiated, and poorly differentiated), chemotherapy (yes/no), radiation (yes/no), and surgery (yes/no). We also tested health status in the models, but inclusion did not change parameter estimates by >10%, and thus final models are presented without health status adjustment.
Trend tests were performed by coding the exposure categories as ordinal and treating the variable as linear. Trend tests for parity and age at first birth were first conducted including all women and then excluding nulliparous women; trend results did not differ substantially. Because there may be unaccounted for factors that distinguish the nulliparous women from those women who have children, we performed additional analyses comparing women who never gave birth to those who did.
We also tested associations between the five reproductive factors of interest and mortality stratified by MHT use to test for residual confounding by MHT use and to further explore interaction that was observed in analyses of reproductive factors and CRC incidence in this cohort (Zervoudakis et al, 2011). We created additional models with more detailed covariates on MHT type (oestrogen only, progestin only, and oestrogen plus progestin) and duration (<5, 5–9, and 10+ years) in the subset of 1245 women with data from the risk factor questionnaire. We also performed analyses stratified by median diagnosis age, median BMI, cancer site (colon or rectum), status of ovaries (both ovaries removed, both ovaries intact, or other surgery to ovaries), and natural menopause (yes/no). We created interaction terms for these covariates with the exposure of interest and tested for statistical significance using the Wald test. We tested the proportional hazards assumption by including an interaction term between person-time and the exposure of interest, and used the Wald test to determine statistical significance (all P-interaction values >0.10). Statistical analyses were performed using SAS 9.3 (SAS Institute Inc. Cary, NC, USA).
Results
The median time from baseline questionnaire to diagnosis was 5.3 years and median follow-up time was 7.7 years. Women who died were more likely to have regional/distant stage tumours, poorly differentiated tumours, and to have received chemotherapy as first course of treatment (Table 1). Women who died were also more likely to be physically inactive, current smokers at baseline, and report worse health status. A greater percentage of women who died reported never use of MHT and history of diabetes.
We observed a suggested, but not statistically significant, lower risk of all-cause mortality among women who were 13+ years old at menarche compared with women who were ⩽12 years old (HR=0.88, 95% CI 0.76–1.01); No overall associations were found for age at menopause (regardless of hysterectomy/oophorectomy status), age at first live birth, parity, or OC use (Table 2). For CRC mortality, we found no associations between these five reproductive or menstrual factors in our analysis. Additional analyses comparing nulliparous to parous women showed no significant associations with mortality.
Compared with women who reported never use of MHT, former MHT use was not associated with all-cause mortality (HR=1.13, 95% CI 0.89–1.43). We also found no association with CRC-specific death among former MHT users (HR=0.98, 95% CI 0.68–1.43) compared with never MHT users. However, among women who reported current MHT use at baseline, we observed a 21% lower risk of all-cause death (HR=0.79, 95% CI 0.66–0.94) and a 24% lower risk of CRC death (HR=0.76, 95% CI 0.59–0.99).
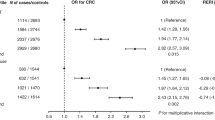
Analyses of OC use, age at menarche, age at first birth, parity, and age at menopause stratified by never, former, or current MHT use showed no statistically significant interactions with all-cause or CRC-specific death (all P-values >0.1; Figures 1 and 2). Interaction terms were also not significant regardless of whether nulliparous women were included in the models.
Reproductive factors and risk of all-cause mortality, stratified by baseline use of hormone replacement therapy. Categories for age at menarche, age at first birth, parity, and age at menopause were ranked in an ordinal fashion and treated as linear; for age at first birth and parity nulliparous women were excluded from models. Models were adjusted for years from questionnaire to diagnosis, body mass index, marital status, smoking status, diabetes, physical activity, tumour stage, tumour grade, chemotherapy, radiation, and surgery.
Reproductive factors and risk of CRC mortality, stratified by baseline use of hormone replacement therapy. Categories for age at menarche, age at first birth, parity, and age at menopause were ranked in an ordinal fashion and treated as linear; for age at first birth and parity, nulliparous women were excluded from models. Models were adjusted for years from questionnaire to diagnosis, body mass index, marital status, smoking status, diabetes, physical activity, tumour stage, tumour grade, chemotherapy, radiation, and surgery.
In analyses stratified by cancer site (colon or rectum), none of the P-interaction values for the examined exposures were significant at a P<0.05 level (Table 3). However, for women with colon cancer, stratified models suggested a non-statistically significant increased risk of CRC-specific death (HR=1.58, 95% CI 0.92–2.70) comparing women age 30+ years at first birth with women who gave birth before 20 years of age. This association was inverse, but not statistically significant, among women with rectal cancer (HR=0.38, 95% CI 0.10–1.40).
There also appeared to be differences in associations by cancer site for MHT use and mortality. Among women with colon cancer, former and current use compared with never use were not associated with mortality. However, among women with rectal cancer, compared with never users, we found no association for former users, but among current users, we observed a significant, 39% lower risk of all-cause death (HR=0.61, 95% CI 0.43–0.87) and a 52% lower risk of CRC death (HR=0.48, 95% CI 0.25–0.92). However, multiplicative interaction was not statistically significant by cancer site (Pinteraction=0.980 for all-cause mortality and Pinteraction=0.531 for CRC mortality).
Among the subset of women with information on MHT preparation, the inverse associations appeared to be stronger among women using oestrogen only compared with women using combined oestrogen–progestin therapy, although smaller numbers may have led to a lack of statistical significance (Table 4). To assess whether tumour stage and grade differed by MHT usage status, we cross-tabulated the factors and found that the percentage of never, former, and current MHT users were similar by both tumour stage and grade.
We did not find evidence of interaction by median diagnosis age (69.8 years), median BMI (26.2 kg m−2), status of ovaries, or natural menopause status (all P-values >0.1).
Discussion
In this study of 2 053 women with CRC, reproductive and menstrual factors were not associated with mortality, while current, but not former, baseline MHT use was associated with lower all-cause and CRC mortality risks.
A previous analysis in this cohort that examined reproductive history and CRC incidence reported increased risks with older age at menopause (55+ vs <40 years old, HR=1.50, 95% CI 1.23–1.83) and age at first birth (30+ vs ⩽19 years old, HR=1.26, 95% CI 1.01–1.58); an inverse association between age at menarche (15+ vs 11–12 years old, HR=0.73, 95% CI 0.57–0.94) and lower risk of CRC was observed only among those women with no history of MHT use (Zervoudakis et al, 2011). We did not find evidence of these patterns for mortality overall or due to CRC. The previous findings in this cohort, in combination with other prospective studies on age at first birth and CRC risk, are equivocal, as two previous studies showed no association (Tamakoshi et al, 2004; Lin et al, 2007), and a third study showed a statistically significant increased risk of developing CRC with older age at first birth (Martinez et al, 1997). One hypothesis for the observed association between reproductive factors and CRC risk is that pregnancy reduces bile acid synthesis, which affects carcinogenesis (McMichael and Potter, 1980). To our knowledge, previous studies have not examined these reproductive factors and mortality among CRC survivors.
Previous studies on MHT and CRC survival have reported variable results. Some studies have shown a ∼30% lower mortality risk among women reporting oestrogen-only therapy, but not among women using combined oestrogen plus progestin therapy (Persson et al, 1996; Chan et al, 2006). The Nurses’ Health Study found 36% lower risk among current as opposed to never users, which differed by duration of use; current use <5 years was associated with a mortality HR (95% CI) of 0.39 (0.23–0.67), whereas current use for 5+ years was nonsignificant (0.83, 0.58–1.18; Chan et al, 2006). Approximately 75% of the NHS study population reported use of oestrogen alone, whereas 25% reported oestrogen plus progestin use, but MHT type-stratified analyses were not presented. Two other studies without information on type of MHT have shown an ∼40% lower risk of mortality among MHT users compared with non-users (Slattery et al, 1999; Mandelson et al, 2003). In contrast, a study in the Women’s Health Initiative (111 CRC cases) showed no association between conjugated equine oestrogen use and CRC mortality (Ritenbaugh et al, 2008). Our findings on MHT use and survival are generally consistent with those found in the Nurses’ Health Study, suggesting that use of MHT is associated with lower CRC mortality. Still, it is possible that lower mortality among MHT users could be due to better health surveillance of these women.
Mechanistic data support a role for endogenous oestrogen in CRC tumorigenesis and prognosis (Sato et al, 2009). In vitro studies have shown that oestrogen affects cell growth in colon cancer cell lines (Singh et al, 1994), and that oestrogen receptor-β (ER-β) protein expression is lower in malignant colon tissue (Foley et al, 2000; Barzi et al, 2013). A review of oestrogen-related molecular pathways and CRC suggested that the loss of ER-β expression during tumorigenesis can be countered by oestrogen ligands, MHT, or soy products, which perhaps may explain the importance of recent timing of hormone use in the protective association with CRC survival (Barzi et al, 2013). Previous studies in humans have also shown increased insulin sensitivity in response to oral equine oestrogens or transdermal E2 patches (Lindheim et al, 1994).
Strengths of this study include the prospective collection of data, which minimises recall bias. Our large sample size permitted separate examination of colon and rectal cancers to identify whether associations with these hormonal and menstrual factors differed by cancer site. We also had information on numerous other CRC risk and prognostic factors, as well as CRC tumour characteristics and treatment data. Limitations of this study include that only a subset of the cohort had detailed information on type of MHT, precluding detailed analyses of both type and duration of use. Also, in our study, we had only a single assessment of MHT use, which may have changed over the follow-up period.
Future studies may seek to further differentiate between mortality risk associated with specific MHT preparations, and further focus on the mechanisms through which recent oestrogen exposures may have a role in colorectal tumour progression.
Change history
28 July 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
American Cancer Society (2014) Cancer Facts and Figures 2014. American Cancer Society: Atlanta, GA, USA.
Barzi A, Lenz AM, Labonte MJ, Lenz H-J (2013) Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res 19 (21): 5842–5848.
Brenner H, Hoffmeister M, Arndt V, Haug U (2007) Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer 96 (5): 828–831.
Chan JA, Meyerhardt JA, Chan AT, Giovannucci EL, Colditz GA, Fuchs CS (2006) Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol 24 (36): 5680–5686.
Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW (2000) Selective loss of estrogen receptor β in malignant human colon. Cancer Res 60 (2): 245–248.
Fritz AG (2000) International Classification of Diseases for Oncology: ICD-O. World Health Organization.
Grodstein F, Newcomb PA, Stampfer MJ (1999) Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med 106 (5): 574–582.
Hermansen SW, Leitzmann MF, Schatzkin A (2009) The impact on National Death Index ascertainment of limiting submissions to Social Security Administration Death Master File matches in epidemiologic studies of mortality. Am J Epidemiol 169: 901–908.
Jacobsen BK, Vollset SE, Kvåle G (1995) Do reproductive factors influence colorectal cancer survival? J Clin Epidemiol 48 (9): 1119–1122.
Lin J, Zhang SM, Cook NR, Manson JE, Buring JE, Lee I-M (2007) Oral contraceptives, reproductive factors, and risk of colorectal cancer among women in a prospective cohort study. Am J Epidemiol 165 (7): 794–801.
Lindheim SR, Duffy DM, Kojima T, Vijod MA, Stanczyk FZ, Lobo R (1994) The route of administration influences the effect of estrogen on insulin sensitivity in postmenopausal women. Fertil Steril 62 (6): 1176–1180.
Mandelson MT, Miglioretti D, Newcomb PA, Harrison R, Potter JD (2003) Hormone replacement therapy in relation to survival in women diagnosed with colon cancer. Cancer Causes Control 14 (10): 979–984.
Martinez ME, Grodstein F, Giovannucci E, Colditz GA, Speizer FE, Hennekens C, Rosner B, Willett WC, Stampfer MJ (1997) A prospective study of reproductive factors, oral contraceptive use, and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 6 (1): 1–5.
McMichael AJ, Potter JD (1980) Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis. J Natl Cancer Inst 65 (6): 1201–1207.
Michaud D, Midthune D, Hermansen S, Leitzmann M, Harlan L, Kipnis V, Schatzkin A (2005) Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registry Manag 32 (2): 70–75.
Newcomb PA, Chia VM, Hampton JM, Doria-Rose VP, Dietz AT (2009) Hormone therapy in relation to survival from large bowel cancer. Cancer Causes Control 20 (4): 409–416.
Persson I, Yuen J, Bergkvist L, Schairer C (1996) Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy—long-term follow-up of a Swedish cohort. Int J Cancer 67 (3): 327–332.
Ritenbaugh C, Stanford JL, Wu L, Shikany JM, Schoen RE, Stefanick ML, Taylor V, Garland C, Frank G, Lane D (2008) Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev 17 (10): 2609–2618.
Writing Group for the Women's Health Initiative Investigators, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288 (3): 321–333.
Sato R, Suzuki T, Katayose Y, Miura K, Shiiba K, Tateno H, Miki Y, Akahira J, Kamogawa Y, Nagasaki S (2009) Steroid sulfatase and estrogen sulfotransferase in colon carcinoma: regulators of intratumoral estrogen concentrations and potent prognostic factors. Cancer Res 69 (3): 914–922.
Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS (2001) Design and Serendipity in Establishing a Large Cohort with Wide Dietary Intake Distributions The National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154 (12): 1119–1125.
Singh S, Paraskeva C, Gallimore PH, Sheppard MC, Langman MJ (1994) Differential growth response to oestrogen of premalignant and malignant colonic cell lines. Anticancer Res 14 (3A): 1037–1041.
Slattery ML, Anderson K, Samowitz W, Edwards SL, Curtin K, Caan B, Potter JD (1999) Hormone replacement therapy and improved survival among postmenopausal women diagnosed with colon cancer (USA). Cancer Causes Control 10 (5): 467–473.
Tamakoshi K, Wakai K, Kojima M, Watanabe Y, Hayakawa N, Toyoshima H, Yatsuya H, Kondo T, Tokudome S, Hashimoto S (2004) A prospective study of reproductive and menstrual factors and colon cancer risk in Japanese women: findings from the JACC study. Cancer Sci 95 (7): 602–607.
Zervoudakis A, Strickler HD, Park Y, Xue X, Hollenbeck A, Schatzkin A, Gunter MJ (2011) Reproductive history and risk of colorectal cancer in postmenopausal women. J Natl Cancer Inst 103 (10): 826–834.
Acknowledgements
This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health, Trenton, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, State Health Division, State of Nevada Department of Health and Human Services, Las Vegas, Nevada. We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management, and Leslie Carroll at Information Management Services for data support and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Arem, H., Park, Y., Felix, A. et al. Reproductive and hormonal factors and mortality among women with colorectal cancer in the NIH-AARP Diet and Health Study. Br J Cancer 113, 562–568 (2015). https://doi.org/10.1038/bjc.2015.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.224
Keywords
This article is cited by
-
Association of hormone replacement therapy with mortality in colorectal cancer survivor: a systematic review and meta-analysis
BMC Cancer (2019)
-
The influence of hormone therapies on colon and rectal cancer
European Journal of Epidemiology (2016)