Abstract
Background:
Patients with human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma (OPSCC) have a better prognosis than those with HPV-negative tumours. There is interest in de-escalating their treatment but strategies are needed for risk stratification to identify subsets with a poor prognosis. This study investigated tumour-infiltrating lymphocytes (TILs) in relation to HPV tumour status and patient survival.
Methods:
Biopsies from 218 patients diagnosed with OPSCC between 2002 and 2011, who underwent chemo/radiotherapy were analysed for HPV by PCR, in-situ hybridisation and p16 immunohistochemistry (IHC). One hundred and thirty-nine samples with concordant HPV detection were analysed for CD3, CD4, CD8 and FoxP3 expression in tumour and stromal regions using multiplexIHC and multispectral image analysis. Labelling of smooth muscle actin (SMA) identified activated stroma.
Results:
Human papillomavirus-positive compared with HPV-negative OPSCC had higher infiltration in both tumour and stromal areas of CD4 and CD8 T cells but not FoxP3 T regulatory cells. Only CD3+CD8+ stromal and not tumour area infiltration was associated with increased survival (P=0.02). There was significantly higher SMA expression in HPV-positive compared with -negative tumours, which did not correlate with survival.
Conclusions:
Studies of TILs for risk stratification in OPSCC should assess stromal infiltration.
Similar content being viewed by others
Main
At present, there is an evolving dichotomy in the landscape of oropharyngeal squamous cell carcinoma (OPSCC) with human papillomavirus (HPV) emerging as an important risk factor for the development of the disease. Conversely, the influence of traditional risk factors (smoking and alcohol) and incidence of HPV-negative OPSCC is decreasing (Sturgis and Cinciripini, 2007). Human papillomavirus-positive and -negative OPSCC have different molecular and clinical features (Fakhry et al, 2008; Cancer Genome Atlas, 2015), with HPV-positive disease having a clearly established better prognosis irrespective of treatment type. This difference is likely to involve multiple factors including differential radio/chemosensitivity and genetic heterogeneity (Ang et al, 2010; Kimple et al, 2013). Human papillomavirus-positive OPSCC are found more frequently at the tonsil and tongue base sub-sites in the oropharynx, but it is unclear what factors underlie this relationship. It is possible that activation of local oropharyngeal immunity has a role in limiting the spread of the disease and/or enhancing response to therapy.
A predominant infiltration of CD3+ T lymphocytes is associated with a favourable prognosis in several cancer types (Gooden et al, 2011). However, the type and functional status of the immune cells (e.g., CD8+ cytotoxic effector vs tumour promoting CD4+FoxP3+ T regulatory (Treg) cells) and/or the micro-environment localisation of different tumour-infiltrating lymphocytes (TILs) can determine the balance between control or promotion of cancer (Fridman et al, 2012). Recent OPSCC TIL studies have produced inconsistent findings. Studies of 46 (Wansom et al, 2012) and 83 (Nasman et al, 2012) patients reported T-cell infiltration was associated with a good prognosis and the degree of T-cell infiltration did not differ by HPV status. By contrast, other studies reported that higher CD8+ but not CD4+ T-cell infiltration was associated with a good prognosis and the degree of infiltration was positively related to HPV status (Nordfors et al, 2013; Ward et al, 2013). Ward et al (2013) investigated 270 OPSCC and found significant differences in T-cell infiltration between HPV-positive and -negative tumours with higher levels associated with a favourable outcome in HPV-positive patients.
Oropharyngeal squamous cell carcinoma have both tumour and stromal elements, which may interact to influence the biology of the cancer. Activation of the tumour stroma may drive tumour progression metabolically and/or influence inflammatory responses by modulating the balance of negative and positive immune-controlling processes (Meseure et al, 2014). Interestingly, activation of the OPSCC stroma determined by smooth muscle actin (SMA) expression was associated with a poor prognosis, although the relationship with HPV status was not determined (Marsh et al, 2011). The stromal extracellular matrix can influence anti-tumour immunity by controlling the positioning and migration of T cells as seen in human lung tumours (Salmon and Donnadieu, 2012). A recent study of OPSCC reported that high CD8+ T-cell infiltration in the stroma was associated with a good prognosis, although, surprisingly, HPV status did not predict a better clinical outcome in this group of patients (Balermpas et al, 2013).
There is currently interest in de-escalating the treatment of good-prognosis OPSCC. Stratification based on HPV status alone may be too simplistic and underpins the need to identify additional biomarkers of outcome. Measurement of TILs is considered a promising avenue of research but requires increased understanding of the subtleties of tumour immunologic response (Jones, 2014). Therefore, the aim of the study reported here was to investigate the role of tumour microenvironment site of T-cell infiltration. The aim was addressed by investigating OPSCC from patients who were diagnosed between 2002 and 2011, and received radiotherapy. Tumour HPV status was determined using three methods and multiplex immunohistochemistry (IHC) was used to detect and enumerate different T-cell populations in tumour and stromal regions.
Materials and methods
Patients
A retrospective audit using a radiotherapy database at The Christie NHS Foundation Trust Hospital identified patients with a confirmed histological diagnosis of OPSCC. Patients were treated between January 2002 and December 2011 with radiotherapy as one or the only therapy modality. Patients treated with a palliative intent were excluded. Patient clinico-pathologic and outcome data were collected from the case notes and The Christie Head and Neck assessment forms. The study was approved by the National Health Service (NHS) National Research Ethics Service committee North West (reference number 03/TG/076). Individual patient consent was not required. Pretreatment formalin-fixed paraffin-embedded blocks prepared at biopsy were requested.
P16 expression
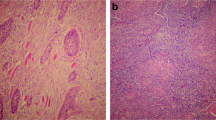
Tumour sections were stained with haematoxylin and eosin to confirm tumour presence before assessing HPV status. Detection of p16INK4a used the CINtec histology kit (Roche, Basel, Switzerland) and a Biogenix i6000 autostainer (Biogenix, Fremont, CA, USA). Human papillomavirus-positive and -negative controls were included in each staining batch. Tumours were scored as positive if there was a strong and diffuse brown staining of the nucleus and cytoplasm in ≥70% of the tumour specimen (Singhi and Westra, 2010). The slides were scored twice by a single scorer with 91% concordance. Any discrepant slides were evaluated by a pathologist to provide a final score.
In-situ hybridisation
The in-situ hybridisation (ISH) assay (Ventana Medical Systems, Tucson, AZ, USA) was performed at Manchester Royal Infirmary according to the manufacturer’s guidelines using the BenchMark automated slide-staining system. The Inform HPV III probe sets were able to detect oncogenic HPV 16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68 and 70. Human papillomavirus DNA ISH slides were scored by two independent observers (80% concordance) and any discrepancies resolved by re-evaluation. A positive score was awarded only for punctate, blue-coloured staining within the nuclei of tumour cells. Diffuse staining of the nuclei was scored as a negative result.
Human papillomavirus DNA PCR
Human papillomavirus DNA-positive samples (by SPF10 DEIA) were genotyped using the INNO-LiPA HPV genotyping assays and the Roche Linear Array HPV genotyping test performed at the Institute of Cancer and Genetics, School of Medicine at Cardiff University, according to protocol.
Multiplex TIL IHC
Formalin-fixed paraffin-embedded sections 4 μm in thickness were deparaffinised in xylene and rehydrated through graded concentrations of alcohol. Following epitope retrieval (HIER) in a pressure cooker, slides were placed in a Biogenex i6000 autostainer and endogenous peroxidases blocked using 3% peroxide solution (ACROS Organics, Geel, Belgium). The first multiplex staining step was DAB visualisation (brown) of FoxP3+ antigen. Protein block was achieved using a 10% casein solution (Vector Laboratories, Burlingame, CA, USA) before staining with the primary antibody (FoxP3, clone 236A/E7 mouse monoclonal antibody (mAb); Abcam, Cambridge, UK; 1:40). Secondary anti-mouse EnVision HRP detection system (Dako, Cambridge, UK) was subsequently used for DAB visualisation. In between stains, slides were transferred to high pH antigen retrieval solution and microwaved at 98 °C for 10 min. For the second and third stains, following peroxidase and casein blocking, CD3 (mouse mAb, clone F7.2.38 (Dako), 1: 60) and CD4 (mouse mAb, clone 4B12 (Dako), 1:50) were visualised with the HRP chromogens ImmPACT VIP SK-4605 (purple; Vector Laboratories) and Vector SG (blue–grey; Vector Laboratories). The fourth step was Vector Red SK-5100 visualisation (red; Vector Laboratories) of CD8 antigen. Following blocking with normal horse serum solution, CD8 antibody (mouse mAb clone C8/144B (Dako), 1:60) was applied and secondary anti-mouse Ig alkaline phosphatase (ImmPRESS, Vector Laboratories) was subsequently used to visualise Vector red. Mouse IgG1 (Dako) was used as a negative control. Finally, following multiplex IHC, sections were counterstained with haematoxylin, dehydrated in graded concentrations of alcohol and coverslipped in permanent, non-aqueous mountant.
Staining for SMA
Sections were cut and deparaffinised, and antigens retrieved as described above. This was followed by a peroxidase block and staining with primary antibody (mouse mAb anti-human SMA clone 1A4 (Dako), 1:50) or negative control reagent, visualisation reagent and substrate-chromogen solution (DAB). Staining was scored using computer-automated H-scores (see below) and by percentage positivity from 30 randomly selected stromal regions in the single slides. Mean scores for each tumour were calculated.
Multiplex IHC automated image analysis and scoring
For each slide, the Vectra automated multispectral imaging system (PerkinElmer, Waltham, MA, USA) was used to perform both low (× 4) and high ( × 20) power scans of 30 randomly selected tissue grids. Spectral libraries were generated from single stained slides using the Nuance FX multispectral imaging system software (PerkinElmer). Spectral libraries and the scanned multiplex images were then loaded into inForm Advanced image analysis software (PerkinElmer). The image analysis method illustrated in Figure 1 comprised the following: (a) resolution of spectral properties of the multiplex stains; (b) classification of regions as tumour, stroma and/or blank spaces; (c) identification of different cell types; and (d) scoring based on the constituent spectral properties. The individual biopsy TIL density/region of interest (ROI; 3.5 × 105 nm2) was determined from 30 randomly selected ROIs of tumour or stromal areas for each section. The mean scores for the whole biopsy, tumour and stroma were generated. The median T-cell density for each group was used to stratify patients into ‘high’ or ‘low’ TIL groups. Automated image analysis was used to quantify the percentage of stromal cells with positive SMA expression (percentage positivity). The staining intensity was classified as low, medium or high, and H-scores were generated by multiplying percentage positivity and staining intensity scores.
Illustration of the method for image analysis of the multiplex immunohistochemically stained sections. The steps involved: image acquisition and processing to obtain the actual multiplex stained image (A); composite imaging of stromal (green), tumour (red) and blank (blue) spaces (B); superimposing A and B (C); enumeration of cells using haematoxylin (D); classification of the quantified cells based on the spectral properties and multiplex staining for tumour infiltrating lymphocytes (E); and quantification of the different T-cell populations in the different compartments as CD3+ (red), CD3+CD4+ (yellow), CD4+ (green) or negative for both markers (blue, F).
Data analysis
Image analysis data were exported to Microsoft Excel worksheets. Charts and data comparisons were performed using SPSS version 20.0 (IBM, Portsmouth, UK) and GraphPad Prism (GraphPad Software, La Jolla, CA, USA). For the box plots shown, Shapiro–Wilk normality test was used to test for the distribution of the data. As the data were mostly not normally distributed, the Mann–Whitney test for comparing the data was used. Actuarial calculations of locroregional control (LRC) and overall survival (OS) were obtained using the Kaplan–Meier method. Univariate analysis was compared using the log rank (Mantel–Cox) method. The χ2-test was used to compare categorical data and the threshold for statistical significance was 0.05.
Results
Patient characteristics
Formalin-fixed paraffin-embedded tumour pretreatment biopsies were available from 218 OPSCC patients. Of these, 139 biopsies showed congruency of HPV-positive or -negative phenotyping by all three detection methods and had sufficient material for further analysis. Concordance rates for HPV detection or non-detection by the three methods was 78%. Supplementary Figure S1 is the consort diagram for the study. The 5-year LRC and OS rates for the cohort were 66% and 52%, respectively. The univariate survival analysis of the patient cohort stratified by different clinico-pathological features is summarised in Supplementary Table S1. Smoking, pretreatment haemoglobin, tumour stage, differentiation and HPV status were associated with LRC and OS. Figure 2 also shows the better LRC (P=0.004) and OS (P=0.0003) of HPV-positive compared with -negative tumours.
Infiltration of T cells
The mean density of CD3+ T cells in the 139 biopsies (both tumour and stromal areas) was 5.2 × 103 per ROI of which 60% (3.1 × 103) were CD4+ and 29% (1.5 × 103) CD8+; only 0.02% (0.06 × 103) of the CD4+ TILs were FoxP3+. Human papillomavirus-positive compared with -negative OPSCC had significantly more CD3+ (P<0.0001), CD3+CD4+ (P<0.0001) and CD3+CD8+ (P<0.0001) but not CD4+FoxP3+ (P=0.1) TILs (Table 1 and Figure 3). A similar pattern of significantly increased infiltration of CD3+, CD3+CD4+ and CD3+CD8+ but not CD4+FoxP3+ T cells was seen in both tumour and stromal regions of HPV-positive compared with -negative OPSCC (Table 1 and Figure 4). There were 9.3-fold more CD3+CD8+ T cells in HPV-positive compared with -negative stromal regions of OPSCC (Table 1).
Scatter plots showing the differential infiltration of the T-cell subsets in HPV-positive ( n =74) and -negative ( n =65) oropharyngeal tumours. CD3+ (A), CD3+CD8+ (B) and CD3+CD4+ (C) had significantly higher infiltration of tumour-infiltrating lymphocytes in HPV-positive cancers. There was no difference in CD4+FoxP3+ cells (D).
Scatter plots of the differential infiltration of T-cell subsets in the various compartments (tumour and stroma) stratified according to HPV status. CD3+ (A), CD3+CD8+ (B) and CD3+CD4+ (C) T cells showed a consistent significantly higher infiltration in the tumour and stromal compartments of HPV-positive tumours. CD4+FoxP3+ (D) showed no difference in T-cell infiltration in the different compartments according to HPV status.
TILs and clinical outcome
There was no difference in OS and LRC for the 139 patients when stratified by median CD3+ TIL density (Figure 5A). Supplementary Table S2 shows the distribution of patients according to high vs low overall CD3+ TIL density. Infiltration was significantly lower in higher American Joint Committee on Cancer prognostic staged tumours (P=0.02) and HPV-negative tumours (P=0.02; Supplementary Table S2). Sub-stratifying by HPV status showed that a high CD3+ T-cell density was associated with significantly better OS in HPV-positive (P=0.02) but not in HPV-negative (P=0.31) patients (Figure 5B and C). There was no statistically significant difference in LRC for high vs low CD3+ T-cell infiltration in either HPV-positive or -negative OPSCC. Further analysis of HPV-positive OPSCC indicated that higher CD3+ T-cell infiltration in the stroma (LRC, P=0.03; OS, P=0.01) but not the tumour (LRC, P=0.75; OS, P=0.15) regions (Figure 5D and E). Interestingly, this improved OS (Figure 5) and LRC (Supplementary Figure S2) was related to significant stromal, and not tumour, CD3+CD8+ but not CD3+CD4+ TILs in HPV-positive OPSCC (Supplementary Table S3).
Kaplan–Meier plots of OS of 139 OPSCC in relation to T-cell infiltration and tumour microenvironment compartment. The panels show low vs high CD3+ T-cell infiltration in all 139 (A), 74 HPV-positive (B) and 65 HPV-negative (C) tumours. (D and E) Survival of HPV-positive patients by CD3+ T-cell infiltration in the tumour and stromal compartments, respectively. (F and G) Survival of HPV-positive patients in relation to infiltration of CD3+CD8+ and CD3+CD4+ T cells, respectively, and the significantly better survival seen with higher CD3+CD8 T-cell infiltration (P=0.05). (H and I) Survival of patients with HPV-positive tumours with infiltration of CD3+CD8+ T cells in tumour and stromal compartments, respectively. Stromal infiltration but not tumour infiltration was associated with significantly better survival (P=0.02).
Stromal activation
Stromal SMA expression (Supplementary Figure S3) was significantly greater in HPV-positive compared with -negative tumours (Supplementary Figure S4) but was not associated with clinical outcome (LRC, P=0.34; OS, P=0.79; Supplementary Table S4). There was no relationship between the percentage positivity of SMA expression and stromal CD3+ TIL density in all patients (Supplementary Table S5). However, a significant correlation was seen in HPV-positive but not in -negative OPSCC stroma for CD3+ (P=0.01) and CD3+CD8+ (P=0.0005) T-cell density Supplementary Figure S5.
Discussion
The OPSCC cohort investigated here had similar OS and LRC rates and clinico-pathological prognostic factors as described previously by others (Gillison et al, 2008; Ang et al, 2010; Oguejiofor et al, 2013). To address potential inadequacies of assessing tumour HPV status in published studies, the three most common HPV detection methods (DNA PCR, DNA ISH and p16 IHC) were used and only those patients with concordant results (78%) were included for study. In the 139 patients with concordant results, 53% were classified as HPV positive, a value similar to that reported previously in OPSCC (Hama et al, 2014). It is pertinent to note that differing positivity rates have been reported globally with rates as low as 20% in the Netherlands to as high as 72% in parts of the United States (Rietbergen et al, 2013).
A strength of the study was the use of multiplex IHC and automated image analysis, which allowed for delineation of specific T-cell populations and reproducible/representative quantitative scoring. This approach enabled not only the extraction of more information from a single slide but also the observation of contextual relationships between detected antigens. The ability to localise the different immune cell phenotypes to different compartments thus adds a layer of complexity and novelty to the study of TILs in OPSCC. The study showed that patients with HPV-positive OPSCC with high CD3+CD8+ T-cell infiltration in stromal areas have the best clinical outcome, which highlights the importance of assessing the tumour microenvironment compartment in TIL studies.
Higher TILs were found in HPV-positive compared with -negative OPSCC. This finding agrees with several (Nasman et al, 2012; Nordfors et al, 2013; Ward et al, 2014) but not all (Wansom et al, 2012; Balermpas et al, 2014) studies. Significantly higher densities of CD3+, CD3+CD4+, CD3+CD8+ but not CD4+FoxP3+ T cells were detected in HPV-positive compared with negative OPSCC as a whole in this study. A further key observation was that this significantly higher infiltration was seen in both tumour and stromal regions. It is apparent that evaluation of TIL density should be done in different tumour microenvironments (Fridman et al, 2012). Stromal cells have been reported to secrete cytokines, which contribute to the recruitment of TILs to the tumour micro-environment (Gajewski et al, 2013). It is presumed that the immune cell infiltration into tumours occurs in response to tumour-specific antigens and an attempt to control tumour growth and spread. However, immune-suppressive factors around the tumour periphery and/or expressed by the tumours can also attract Treg cells able to inhibit otherwise potentially active effector T cells. In this study, no difference was seen in the infiltration of Treg (CD4+FoxP3) populations as stratified by HPV status, although the numbers of these cells detected were very small. Indeed, CD4+FoxP3+ T cells were often absent from many of the scanned images (<4 per section) increasing uncertainly about the value of quantitative comparisons. There is evidence by others that high levels of systemic Treg cells are a positive prognostic marker in OPSCC (Khorramirouz et al, 2014; Lukesova et al, 2014). The approach used in this paper of evaluating Treg cells in the local tumour environment did not show any prognostic relationship. It is possible that FoxP3+ may not mark all Treg cells and/or that other immune cells such as macrophages or myeloid derived suppressor cells, which were not assessed, could act to limit CD8+ T-cell activity (Damuzzo et al, 2014; Caronni et al, 2015).
As expected and reported widely, HPV-positive compared with -negative OPSCC patients had a significantly better OS (P=0.0003) and LRC (P=0.0037). In contrast with other reports where T-cell density correlated with survival in both HPV-positive and -negative patients (Nasman et al, 2012; Wansom et al, 2012; Balermpas et al, 2014), no difference was found in outcomes when stratifying all patients by total CD3+ T-cell infiltration. The heterogeneity in the patients with oral cancers studied, robustness of HPV detection and the use of a variety of IHC methods may have contributed to discordance between various published reports. However, T-cell infiltration was significantly associated with survival in HPV-positive but not in -negative OPSCC patients as reported in another study (Ward et al, 2013). In addition, our observations suggest that the stromal infiltration of CD3+CD8+ T cells is important in determining prognosis. This is consistent with a hypothesis that the higher infiltration of CD8+ T cells in the stroma is a marker of an effective immune response in HPV-positive OPSCC contributing to improved outcome following standard therapy. In cervical dysplasia, lesion regression has been associated with T-cell infiltration of the epithelium (Trimble et al 2010), but there is evidence that the stroma is the first point of call for the effector immune cells (Kobayashi et al, 2004; Trimble et al, 2010; Maldonado et al, 2014). Gene analysis of micro-dissected specimen of pre- and post-vaccination dysplastic lesions showed an increase in genes associated with effector immune cell phenotype, polarisation, function and activation in the stroma of post vaccination patients (Maldonado et al, 2014).
In addition to TILs, the activation status of the stroma was investigated using SMA expression by activated myofibroblasts. When present at sites of chronic inflammation, myofibroblasts promote angiogenesis, extracellular matrix, growth factor and cytokine expression (Surowiak et al, 2007). Smooth muscle action expression has been associated with a poor prognosis in several cancer types. In patients with SCC of the tongue, Kellermann et al (2007) observed higher SMA expression at the invasive margin significantly correlated with invasion into blood vessels, lymph node and neurons. Stromal features predicted disease mortality in oral cancer, although the proportion of OPSCC and HPV positivity was not stated (Marsh et al, 2011). Our results showed a significantly higher expression of SMA in the stroma of HPV-positive compared with -negative OPSCC, but there was no association with survival. The differing numbers, heterogeneity of pathology, therapy and follow-up of patients with oral cancers studied, robustness of HPV detection and variations in IHC methodology may all have contributed to the discordance between the various published reports.
There are further levels that can influence immune control in cancer patients through co-stimulatory inhibitory pathways. The programmed cell death protein 1 (PD-1) is expressed on activated T cells and its ligands PD-L1 and PD-L2 on antigen-presenting cells (APCs) and sometimes tumour cells. The infiltration of PD-1-expressing lymphocytes can be a marker of favourable prognosis in HPV-positive OPSCC (Badoual et al, 2013) but localisation of PD-L1 to the tumour stroma interface or other immune cell types (e.g., macrophages) might limit functional tumour infiltration (Lyford-Pike et al, 2013). Trials investigating the blockade of PD-1 receptors in a range of cancers (melanoma, colorectal cancer, non-small-cell lung cancer and renal cancer) have shown clinical activity (Gubin et al, 2014; Herbst et al, 2014; Powles et al, 2014; Tumeh et al, 2014). In this study, the relationship between tumour micro-environment, expression of PD1, PDL1 and PDL2 by TILs, tumour cells and/or APC was not evaluated. It is hypothesised that the presence of TIL in the tumour compartment maybe subject to the suppressive effects of the co-stimulatory factors. Future studies using a multiple marker approach could investigate this relationship.
In summary, there is a growing body of evidence for an underlying immune activity in OPSCC, which is more important for HPV-positive compared with -negative disease. The work reported here highlights the importance of evaluating its context-specific nature in relation to the immune cell localisation and functional orientation. As stated in the Introduction section, the move towards patient stratification based on HPV status alone may be too simplistic, with measurements of TILs considered a promising avenue for biomarker development (Jones, 2014). Our work supports this suggestion but, in addition, shows the need to assess microenvironment localisation.
Change history
15 September 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363 (1): 24–35.
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, Gey A, Rotem-Yehudar R, Pere H, Tran T, Guerin CL, Chauvat A, Dransart E, Alanio C, Albert S, Barry B, Sandoval F, Quintin-Colonna F, Bruneval P, Fridman WH, Lemoine FM, Oudard S, Johannes L, Olive D, Brasnu D, Tartour E (2013) PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 73 (1): 128–138.
Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, Rodel C, Fokas E (2014) Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer 110 (2): 501–509.
Balermpas P, Michel Y, Wangenblast J, Seitz O, Weiss C, Rodel F, Rodel C, Fokas E (2013) Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer 110 (2): 501–509.
Cancer Genome Atlas N (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517 (7536): 576–582.
Caronni N, Savino B, Bonecchi R (2015) Myeloid cells in cancer-related inflammation. Immunobiology 220 (2): 249–253.
Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S (2014) Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin Cytom ; e-pub ahead of print 26 November 2014; doi:10.1002/cytob.21206.
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100 (4): 261–269.
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12 (4): 298–306.
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14 (10): 1014–1022.
Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100 (6): 407–420.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105 (1): 93–103.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD (2014) Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515 (7528): 577–581.
Hama T, Tokumaru Y, Fujii M, Yane K, Okami K, Kato K, Masuda M, Mineta H, Nakashima T, Sugasawa M, Sakihama N, Yoshizaki T, Hanazawa T, Kato H, Hirano S, Imanishi Y, Kuratomi Y, Otsuki N, Ota I, Sugimoto T, Suzuki S (2014) Prevalence of human papillomavirus in oropharyngeal cancer: a multicenter study in Japan. Oncology 87 (3): 173–182.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515 (7528): 563–567.
Jones TM (2014) Tumour-infiltrating lymphocytes in the risk stratification of squamous cell carcinoma of the head and neck. Br J Cancer 110 (2): 269–270.
Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, Nishimoto I, Kowalski LP, Coletta RD (2007) Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology 51 (6): 849–853.
Khorramirouz R, Ebadi M, Rahimi Sherbaf F, Kajbafzadeh AM (2014) Persistent high level of urinary tumor marker carbohydrate antigen 19-9 in prenatally diagnosed dysplastic kidney. Case Rep Urol 2014: 259870.
Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, Peet CR, Lorenz LD, Nickel KP, Klingelhutz AJ, Lambert PF, Harari PM (2013) Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 73 (15): 4791–4800.
Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, Levine AM, Darragh TM, Weinberg V, Smith-McCune KK (2004) Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res 64 (18): 6766–6774.
Lukesova E, Boucek J, Rotnaglova E, Salakova M, Koslabova E, Grega M, Eckschlager T, Rihova B, Prochazka B, Klozar J, Tachezy R (2014) High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. BioMed Res Int 2014: 303929.
Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, Chen L, Drake CG, Topalian SL, Pardoll DM, Pai SI (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73 (6): 1733–1741.
Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL (2014) Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 6 (221): 221ra13.
Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH, Chester KA, Chana JS, Marshall JF, Hart IR, Hackshaw AK, Piper K, Thomas GJ (2011) Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 223 (4): 470–481.
Meseure D, Drak Alsibai K, Nicolas A (2014) Pivotal role of pervasive neoplastic and stromal cells reprogramming in circulating tumor cells dissemination and metastatic colonization. Cancer Microenviron 7 (3): 95–115.
Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T (2012) Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 7 (6): e38711.
Nordfors C, Grun N, Tertipis N, Ahrlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund L, Munck-Wikland E, Nasman A, Ramqvist T, Dalianis T (2013) CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer 49 (11): 2522–2530.
Oguejiofor KK, Hall JS, Mani N, Douglas C, Slevin NJ, Homer J, Hall G, West CM (2013) The prognostic significance of the biomarker p16 in oropharyngeal squamous cell carcinoma. Clin Oncol (R Coll Radiol) 25 (11): 630–638.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515 (7528): 558–562.
Rietbergen MM, Leemans CR, Bloemena E, Heideman DA, Braakhuis BJ, Hesselink AT, Witte BI, Baatenburg de Jong RJ, Meijer CJ, Snijders PJ, Brakenhoff RH (2013) Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer 132 (7): 1565–1571.
Salmon H, Donnadieu E (2012) Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. Oncoimmunology 1 (6): 992–994.
Singhi AD, Westra WH (2010) Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 116 (9): 2166–2173.
Sturgis EM, Cinciripini PM (2007) Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110 (7): 1429–1435.
Surowiak P, Murawa D, Materna V, Maciejczyk A, Pudelko M, Ciesla S, Breborowicz J, Murawa P, Zabel M, Dietel M, Lage H (2007) Occurence of stromal myofibroblasts in the invasive ductal breast cancer tissue is an unfavourable prognostic factor. Anticancer Res 27 (4C): 2917–2924.
Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, Kos F, Teague J, Jiang Y, Barat NC, Jungbluth AA (2010) Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol 185 (11): 7107–7114.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515 (7528): 568–571.
Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, Chepeha D, Kumar B, Cordell K, Eisbruch A, Taylor J, Moyer J, Bradford C, D'Silva N, Carey T, McHugh J, Wolf G (2012) Infiltrating lymphocytes and human papillomavirus-16—associated oropharyngeal cancer. Laryngoscope 122 (1): 121–127.
Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, Webb A, Hampton C, Patel NN, Randall CJ, Cox HJ, Jogai S, Primrose J, Piper K, Ottensmeier CH, King EV, Thomas GJ (2013) Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer 110 (2): 489–500.
Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, Webb A, Hampton C, Patel NN, Randall CJ, Cox HJ, Jogai S, Primrose J, Piper K, Ottensmeier CH, King EV, Thomas GJ (2014) Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer 110 (2): 489–500.
Acknowledgements
The study was supported by Experimental Cancer Medicine Centre funding. Some of the HPV testing work was supported by a grant from GSK for the UK PREVALENCE study (chief investigator Professor Terry Jones). The PCR testing was carried out by Dr Ned Powell and Kate Cushieri for the PREVALENCE study. We thank Helen Valentine and Joely Irlam for laboratory support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Oguejiofor, K., Hall, J., Slater, C. et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer 113, 886–893 (2015). https://doi.org/10.1038/bjc.2015.277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.277
Keywords
This article is cited by
-
HPV-positive murine oral squamous cell carcinoma: development and characterization of a new mouse tumor model for immunological studies
Journal of Translational Medicine (2023)
-
CT radiomic signature predicts survival and chemotherapy benefit in stage I and II HPV-associated oropharyngeal carcinoma
npj Precision Oncology (2023)
-
HPV-associated head and neck cancer is characterized by distinct profiles of CD8+ T cells and myeloid-derived suppressor cells
Cancer Immunology, Immunotherapy (2023)
-
Molecular prognostic indicators in HPV-positive oropharyngeal cancer: an updated review
Clinical & Experimental Metastasis (2022)
-
Tumor microenvironment: an evil nexus promoting aggressive head and neck squamous cell carcinoma and avenue for targeted therapy
Signal Transduction and Targeted Therapy (2021)