Abstract
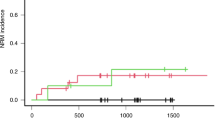
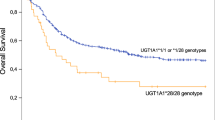
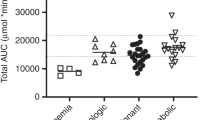
Busulfan (BU) is a key compound in conditioning myeloablative regimens for children undergoing hematopoietic stem cell transplantation (HSCT). There are wide interindividual differences in BU pharmacokinetics, which increase the risk of veno-occlusive disease, graft rejection and disease relapse. As BU is mainly metabolized by glutathione S-transferase (GST), it is hypothesized that functional polymorphisms in GST genes may explain in part the variability in BU pharmacokinetics. We analyzed polymorphisms in GSTA1 (C-69T, A-513G, G-631T, C-1142G), GSTM1 (deletion) and GSTP1 (A1578G, C2293T) genes in 28 children undergoing HSCT. All patients had individualized dosing based on pharmacokinetics after the first dose of intravenous BU. GSTM1-null individuals had higher drug exposure (PCmax=0.008; PAUC=0.003; PCss=0.02) and lower clearance (PCL=0.001). Multivariate regression models showed that, other than the drug dose and age, the GSTM1 genotype was the best predictor of first-dose pharmacokinetic variability. GSTM1-null patients also received lower cumulative BU doses (P=0.02). No association was found between BU exposure and major GSTA1 or GSTP1 gene variants. In children, GSTM1 polymorphism seems to modify BU pharmacokinetics after intravenous drug administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Vassal G, Koscielny S, Challine D, ValteauCouanet D, Boland I, Deroussent A et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–253.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant 2001; 28: 743–751.
Tran HT, Madden T, Petropoulos D, Worth LL, Felix EA, Sprigg-Saenz HA et al. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Bone Marrow Transplant 2000; 26: 463–470.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Dalle JH, Wall D, Theoret Y, Duval M, Shaw L, Larocque D et al. Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results. Bone Marrow Transplant 2003; 32: 647–651.
Czwerwinski M, Gibbs JP, Slattery JT . Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos 1996; 24: 1015–1019.
AliOsman F, Akande O, Antoun G, Mao JX, Buolamwini J . Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants—evidence for differential catalytic activity of the encoded proteins. J Biol Chem 1997; 272: 10004–10012.
Rebbeck TR . Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev 1997; 6: 733–743.
Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics 2001; 11: 663–669.
Guy CA, Hoogendoorn B, Smith SK, Coleman S, O'Donovan MC, Buckland PR . Promoter polymorphisms in glutathione-S-transferase genes affect transcription. Pharmacogenetics 2004; 14: 45–51.
Morel F, Rauch C, Coles B, Le Ferrec E, Guillouzo A . The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics 2002; 12: 277–286.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Rifai N, Sakamoto M, Lafi M, Guinan E . Measurement of plasma busulfan concentration by high-performance liquid chromatography with ultraviolet detection. Ther Drug Monit 1997; 19: 169–174.
Bartelink IH, Bredius RGM, Belitser SV, Suttorp MM, Bierings M, Knibbe CAJ et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 231–241.
Bredschneider M, Klein K, Murdter TE, Marx C, Eichelbaum M, Nussler AK et al. Genetic polymorphisms of glutathione S-transferase A1, the major glutathione S-transferase in human liver: consequences for enzyme expression and busulfan conjugation. Clin Pharmacol Ther 2002; 71: 479–487.
Zhong S, Wyllie AH, Barnes D, Wolf CR, Spurr NK . Relationship between the GSTM1 genetic-polymorphism and susceptibility to bladder, breast and colon-cancer. Carcinogenesis 1993; 14: 1821–1824.
Bourgeois S, Labuda D . Dynamic allele-specific oligonucleotide hybridization on solid support. Anal Biochem 2004; 324: 309–311.
Krajinovic M, Labuda D, Sinnett D . Glutathione S-transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics 2002; 12: 655–658.
Mosteller RD . Simplified calculation of body-surface area. N Engl J Med 1987; 317: 1098.
Stephens M, Smith NJ, Donnelly P . A new statistical method for haplotype reconstruction from population data. Am J Human Genet 2001; 68: 978–989.
Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M . Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol 1993; 33: 181–186.
Kassir N, Theoret DY, Champagne MA, Duval M, Larocque D, Labbe L . Population pharmacokinetic of intravenous busulfan in children. Clin Pharmacol Ther 2007; 81: S81.
Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C . I. V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant 2004; 33: 979–987.
Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y . Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 37: 345–351.
Bartelink IH, Bredius RGM, Ververs TT, Raphael MF, van Kesteren C, Bierings M et al. Once-daily intravenous busulfan with therapeutic drug monitoring compared to conventional oral busulfan improves survival and engraftment in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14: 88–98.
Bleyzac N . The use of pharmacokinetic models in paediatric onco-haematology: effects on clinical outcome through the examples of busulfan and cyclosporine. Fundam Clin Pharmacol 2008; 22: 605–608.
Zwaveling J, Bredius RGM, Cremers S, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Nath CE, Earl JW, Pati N, Stephen K, Shaw PJ . Variability in the pharmacokinetics of intravenous busulphan given as a single daily dose to paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 2008; 66: 50–59.
Zwaveling J, Press RR, Bredius RGM, van der Straaten T, den Hartigh J, Bartelink IH et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Ther Drug Monit 2008; 30: 504–510.
Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood 2004; 104: 1574–1577.
Johnson L, Orchard PJ, Baker KS, Brundage R, Cao Q, Wang XJ et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol 2008; 48: 1052–1062.
Bouligand J, Boland I, Valteau-Couanet D, Deroussent A, Kalifa C, Hartmann O et al. In children and adolescents, the pharmacodynamics of high-dose busulfan is dependent on the second alkylating agent used in the combined regimen (melphalan or thiotepa). Bone Marrow Transplant 2003; 32: 979–986.
Kusama M, Kubota T, Matsukura Y, Matsuno K, Ogawa S, Kanda Y et al. Influence of glutathione S-transferase A1 polymorphism on the phartnacokinetics of busulfan. Clin Chim Acta 2006; 368: 93–98.
Acknowledgements
We are thankful to all the patients and their parents who consented to participate in this HSCT-related genetics study. This work was supported by grants from PDL Biopharma, the Fondation Télémaque and Fondation du centre de cancérologie Charles-Bruneau. MK is a scholar of the Fonds de la Recherche en Santé du Québec. MA is a scholar of the Fondation Télémaque and the Fondation Charles-Bruneau.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansari, M., Lauzon-Joset, JF., Vachon, MF. et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant 45, 261–267 (2010). https://doi.org/10.1038/bmt.2009.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.143
Keywords
This article is cited by
-
Role of Genetic Polymorphisms in Drug-Metabolizing Enzyme-Mediated Toxicity and Pharmacokinetic Resistance to Anti-Cancer Agents: A Review on the Pharmacogenomics Aspect
Clinical Pharmacokinetics (2022)
-
The analysis of GSTA1 promoter genetic and functional diversity of human populations
Scientific Reports (2021)
-
Comparison of Two Analytical Methods for Busulfan Therapeutic Drug Monitoring
European Journal of Drug Metabolism and Pharmacokinetics (2021)
-
Evaluation of two software using Bayesian methods for monitoring exposure and dosing once-daily intravenous busulfan in paediatric patients receiving haematopoietic stem cell transplantation
Cancer Chemotherapy and Pharmacology (2021)
-
Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients
Clinical Pharmacokinetics (2021)