Abstract
Triple-negative breast cancer (TNBC) is a distinct breast cancer subtype defined by the absence of estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (HER2/neu), and the patients with TNBC are often diagnosed with higher rates of recurrence and metastasis. Because of the absence of ER, PR and HER2/neu expressions, TNBC patients are insensitive to HER2-directed and endocrine therapies available for breast cancer treatment. Here, we report that expression of atypical protein kinase C isoform, PKCλ/ι, significantly increased and activated in all invasive breast cancer (invasive ductal carcinoma or IDC) subtypes including the TNBC subtype. Because of the lack of targeted therapies for TNBC, we choose to study PKCλ/ι signaling as a potential therapeutic target for TNBC. Our observations indicated that PKCλ/ι signaling is highly active during breast cancer invasive progression, and metastatic breast cancers, the advanced stages of breast cancer disease that developed more frequently in TNBC patients, are also characterized with high levels of PKCλ/ι expression and activation. Functional analysis in experimental mouse models revealed that depletion of PKCλ/ι significantly reduces TNBC growth as well as lung metastatic colonization. Furthermore, we have identified a PKCλ/ι-regulated gene signature consisting of 110 genes, which are significantly associated with indolent to invasive progression of human breast cancer and poor prognosis. Mechanistically, cytokines such as TGFβ and IL1β could activate PKCλ/ι signaling in TNBC cells and depletion of PKCλ/ι impairs NF-κB p65 (RelA) nuclear localization. We observed that cytokine-PKCλ/ι-RelA signaling axis, at least in part, involved in modulating gene expression to regulate invasion of TNBC cells. Overall, our results indicate that induction and activation of PKCλ/ι promote TNBC growth, invasion and metastasis. Thus, targeting PKCλ/ι signaling could be a therapeutic option for breast cancer, including the TNBC subtype.
Similar content being viewed by others
Main
Breast cancer is a clinically heterogeneous disease and both intra and inter-tumor heterogeneities provide great challenges for developing successful therapies. Expressions (or absence thereof) of estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (HER2)/neu are widely used to clinically classify breast tumors into multiple therapeutic groups.1 The ER/PR-positive and the HER2-positive breast cancer patients could be benefited from endocrine and HER2-targeted therapies.1 However, triple-negative breast cancers (TNBCs), which represent ∼12–17% of all breast cancer,2 lack ER, PR and HER2/neu expressions2 and are not responsive to therapies targeting these receptors. Therefore, the only systemic therapy available for TNBC is chemotherapy.3 Furthermore, TNBC is associated with aggressive pathologic features like higher histology grade and mitotic index4 and often found to be associated with higher rate of metastasis and recurrence leading to limited clinical outcome.5, 6, 7, 8 Recurrence of TNBC tends to recur within a few years after successful initial treatment6, 9 and often develops metastasis to the bone, brain and lungs with poor prognosis.2, 6 Thus, identification of signaling pathways that regulate malignant progression of breast cancer subtypes, especially TNBCs, would be therapeutically important.
In recent years, PKC signaling has been implicated in modulating invasion and metastasis of multiple tumors.10, 11 The PKC family consists of multiple serine/threonine kinases and the relative contribution of individual PKC isoforms during cancer progression varies due to pleiotropism.12 PKC isoforms regulate diverse cellular functions such as cell-cycle regulation, cellular survival, cell–cell communications and apoptosis.13 In particular, atypical PKC isoforms, PKCζ and atypical protein kinase C lamda/iota (PKCλ/ι), are known to be important for chemotaxis, cell polarity, migration and wound healing processes.14, 15 Aberrations in all these processes are manifested in tumor progression and metastasis.14 Consistent with these notions, recent studies indicated that atypical PKCs are associated with various human cancers.10, 11 Importantly, the PKCλ/ι gene is located at the 3q26.2 genomic region, which is most frequently amplified in human cancer16, 17, and overexpression of PKCλ/ι has been implicated in cancer development in multiple tissues including the lung,18, 19 pancreas,20 stomach,21 colon,22 esophagus,23 liver,24 bile duct,25 ovary,17 prostate26 and brain.27 Recently, few studies have been reported higher expression of PKCλ/ι in ER/PR- and HER-positive breast cancer and also in lymph node metastases.28, 29 Kojima et. al.28 showed that PKCλ/ι expression is highly induced in the ER/PR- and HER2-positive IDCs compared with ductal carcinoma in situ (DCIS) and normal breast. PKCλ/ι forms apical-junctional complexes (AJCs) with other polarity proteins such as partitioning defective 3 homolog (PAR3) and partitioning defective 6 homolog (PAR6),30, 31, 32, 33 and invasiveness of breast tumor cells was shown to be associated with loss of PKCλ/ι localization from their apical domains.28 In addition, predominant nuclear localization of PKCλ/ι in both normal and atypical ductal hyperplasia (ADH) lesions prompted the concept that PKCλ/ι might be in an inactive state in these lesions.28 However, expression and activation of PKCλ/ι in TNBCs and the functional importance of PKCλ/ι signaling in relation to invasive breast cancer progression and metastasis are very poorly understood.10, 11
Here, we studied PKCλ/ι signaling during invasive progression of TNBC. We utilized expression evaluations in triple-negative IDCs as well as metastatic breast cancers of human patients, in vitro and in vivo functional assays, and global gene expression analysis of human patient samples. We concluded that PKCλ/ι signaling is an important regulator for invasion and metastatic progression of human breast cancers including triple-negative subtypes.
Results
PKCλ/ι is highly active in human TNBCs and metastatic breast cancers
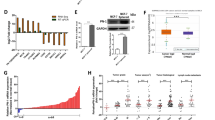
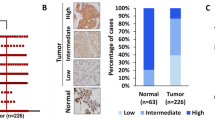
To investigate PKCλ/ι signaling in breast cancer disease progression, we tested expression of PKCλ/ι in human breast sample cohort, consisting of normal breasts, DCIS and IDC samples of ER-positive, HER2-positive and TNBC subtypes (Figures 1a–d, Supplementary Figure S1a and S1b). Immunohistochemical analysis of PKCλ/ι expression indicated significant increase in ER- and HER2-positive IDC samples compared with normal breast and DCIS (Figure 1a, Supplementary Figures S1c and d) and supported previous reports.28, 29 Importantly, we also observed significant higher expression of PKCλ/ι in TNBC subtypes compared with normal breast and DCIS (Figures 1a–c, Supplementary Figures S1c and d). In all IDC samples, the induced PKCλ/ι localization was detected in both cytoplasm and nuclei, (Figure 1d and Supplementary Figure S1b) with a few cases showing focal (5–25%) nuclear staining.
Human breast cancers are associated with higher expression and phosphorylation of PKCλ/ι. Expression of PKCλ/ι (a) and phospho- PKCλ/ι (b) determined by Immunohistochemistry (IHC) in normal breast (n=10), DCIS (n=10) and IDC samples of ER+ subtype (n=10), HER2+ subtype (n=10) and triple-negative subtype (n=35). Results were expressed as IHC scores of individual samples (by two independent pathologists) using a scale 0–3. IHC scores in between 0 to 1 considered as low expression, whereas IHC scores >1 considered as high expression. Expression of PKCλ/ι and phospho-PKCλ/ι in all IDC subtypes were significantly higher compared with DCIS and normal breast. P-values were calculated by two-way ANOVA with Bonferroni post-test (see also Supplementary Figure S1c–f for detailed statistical analysis). (c) Representative images of PKCλ/ι and phospho-PKCλ/ι expression in human normal breast, DCIS and triple-negative IDC samples. Expression of PKCλ/ι and phospho-PKCλ/ι gradually increased from normal breast to DCIS, and further increased in triple-negative IDCs. (d) Representative images showing localization of PKCλ/ι and phospho- PKCλ/ι in ER+, HER2+ and triple-negative IDCs. (e) Expression of PKCλ/ι and phospho-PKCλ/ι in human metastatic breast cancers (n=10)
To test the distribution of activated form of PKCλ/ι, we used a previously validated antibody34, 35, 36 that selectively recognizes PKCλ/ι molecules, phosphorylated at threonine 555 (T555) and threonine 563 (T563) residues in the catalytic domain15, 37 (phospho-PKCλ/ι). We observed that phospho-PKCλ/ι levels were also significantly increased from normal breast to DCIS to IDC samples of all clinical subtypes (Figures 1b–d, Supplementary Figures S1e and f). Interestingly, in contrast to total PKCλ/ι, we observed predominant nuclear localization of phospho-PKCλ/ι in all IDC samples (Figure 1d and Supplementary Figure S1b). As phosphorylation event (specifically at T555) is believed to prime activation of PKCλ/ι,38 our observations indicate predominant localization of active PKCλ/ι in the nuclei of breast tumor cells and oppose the idea that inactive PKCλ/ι localizes within nuclei, as suggested earlier.28
Next, we collected multiple human metastatic breast cancer samples (n=10) from distal organs such as the bone, brain, chest wall, colon, gallbladder, liver, lung and ovary to test PKCλ/ι expression and phosphorylation (Figure 1e). We found that similar to IDC and lymph node metastasis,29 PKCλ/ι is highly expressed and phosphorylated in the metastatic breast cancers with predominant nuclear localization of phospho-PKCλ/ι (Figure 1e). Collectively, these results and previous reports28, 29 strongly correlate higher expression and activation of PKCλ/ι with invasive progression of breast cancer including TNBC subtypes to develop local and distal metastasis.
PKCλ/ι regulates epithelial morphology, invasion and migration of triple-negative breast cancer cells
On the basis of our observation in human patient samples, we hypothesized that PKCλ/ι signaling could promote invasion and metastasis of TNBC. Therefore, we specifically depleted PKCλ/ι in MDA-MB-231 cells, a highly invasive and metastatic TNBC cell line, via RNA interference (RNAi) (Figure 2a and Supplementary Figure S2). We found that specific depletion of PKCλ/ι induced mesenchymal-to-epithelial transition (MET)-like response in MDA-MB-231 cells (Figures 2b and c). Notably, these morphological changes of MDA-MB-231 also associated with significant inhibition in invasion through Matrigel-coated transwells and also migration during wound closure assays (Figures 2d–g). To test whether PKCλ/ι signaling is a common positive regulator of invasion and migration, we depleted PKCλ/ι in multiple TNBC cell lines (Supplementary Figure S3a). We found that depletion of PKCλ/ι significantly inhibited invasion and migration of multiple triple-negative breast cancer cells including MDA-MB-468 (EGFR-expressing and TP53 mutant),39 BT-20 (constitutively active ER carrying deletion of exon 5, EGFR-expressing and TP53 mutant)40 and HCC-1937 (EGFR-expressing, null for p53 expression and homozygous breast cancer 1, early onset (BRCA1) mutation carrier)41 (Supplementary Figures S3b and c).
Depletion of PKCλ/ι signaling promotes MET and inhibits invasion and migration of breast cancer cells. (a) Specific knockdown of PKCλ/ι in MDA-MB-231 cells. (b) Morphologies of PKCλ/ι-depleted MDA-MB-231 cells. (c) Expression of epithelial marker E-Cadherin and basal marker N-Cadherin in PKCλ/ι-depleted MDA-MB-231 cells (n=3). (d) Transwell invasion of PKCλ/ι-depleted MDA-MB-231 cells. Inset represents higher magnification image of the corresponding transwell filter. (e) Quantification of invasion assays (each field was divided into nine unit areas and three fields per condition). (f) Wound closure assays of PKCλ/ι-depleted MDA-MB-231 cells. (g) Quantification of wound closure assays (n=3). For all quantifications, results represent means±S.E.M. P-values were calculated by two-tailed unpaired Student’s t-test. **P≤0.01, ***P≤0.001
We checked survival and proliferation of all the PKCλ/ι-depleted TNBC cells and found no significant difference compared with the controls (Supplementary Figure S3d). These results indicate that specific depletion of PKCλ/ι can inhibit the invasive potential of TNBC cells regardless of their TP53 status, EGFR expression and BRCA1 mutation without affecting their survival in vitro.
PKCλ/ι regulates TNBC growth, invasion and metastasis in vivo
Our in vitro analyses indicated that PKCλ/ι depletion in TNBC cells strongly inhibits their invasive potential (Figure 2 and Supplementary Figure S3). Furthermore, analysis in human patient samples showed that PKCλ/ι expression and phosphorylation are induced in all IDC subtypes including TNBC and metastatic breast cancers (Figure 1). Thus, we tested whether PKCλ/ι signaling could promote TNBC growth and invasion in vivo. We orthotopically transplanted luciferase reporter expressing MDA-MB-231 (MDA-MB-231-luc) cells with or without PKCλ/ι depletion, into the mammary glands of immunodeficient mice (n=5) and monitor tumor growth via bioluminescence imaging (BLI) (Figure 3a). We found that loss of PKCλ/ι resulted in significant inhibition in primary tumor growth within 5 weeks (Figure 3b–f and Supplementary Figure S4). Importantly, similar to our observation in human IDC samples and metastatic breast cancers (Figures 1d and e), we observed that PKCλ/ι predominantly localized in the cytoplasm and phospho-PKCλ/ι predominantly localized in the nuclei of the xenografted MDA-MB-231 cells (Figure 3g).
Depletion of PKCλ/ι inhibits orthotopic breast tumor growth. (a) Schematic of orthotopic breast tumor formation assay with PKCλ/ι-depleted MDA-MB-231-luc cells. (b) Representative whole-animal images at 5 weeks post-transplantation. (c) Quantification of tumor growth via luminescence measurements (n=5). (d) Representative tumors at 5 weeks post-transplantation. Arrow heads indicating tumor regions on second mammary glands. (e) Quantification of tumor area (n=5). (f) H&E staining of breast tissues at 5 weeks post-transplantation. (g) Immunohistochemical analysis of PKCλ/ι and phospho-PKCλ/ι expression and localization in control MDA-MB-231 xenograft breast tumor. For all quantifications, results represent means±S.E.M. P-values were calculated two-way ANOVA with Bonferroni post-test (see also Supplementary Figure S4 for detailed statistical analysis)
Although PKCλ/ι depletion does not induce overt changes of TNBC cell proliferation during in vitro culture conditions (Supplementary Figure S3d), our in vivo results suggest that the growth of primary tumors from orthotopically transplanted TNBC cells was dependent upon a cell-autonomous PKCλ/ι signaling. We reasoned that, during in vitro studies, a plethora of signaling molecules was present in high concentrations in serum-supplemented growth media and these signaling molecules might simultaneously activate multiple signaling cascades, thereby compensating the loss of PKCλ/ι signaling pathway. However, that compensation was absent in vivo as transplanted cells only had limited access to tissue specific growth factors or signaling molecules, which are highly regulated in a spatio-temporal manner within a complex environment and are present at their physiological concentrations.
Along with growth of primary tumors, we also assessed invasive progression of MDA-MB-231 cells in vivo upon PKCλ/ι depletion. We tested spontaneous metastasis of orthotopically transplanted MDA-MB-231 cells with and without PKCλ/ι depletion (n=5). We transplanted cells into the fourth mammary gland of immunodeficient mice, removed primary tumor at 5 weeks and observed for spontaneous metastasis to the lung (Figure 4a). Again, we observed inhibition in orthotopic tumor growth upon PKCλ/ι depletion within 5 weeks (Figures 4b and 4c) and also observed lung metastasis of control MDA-MB-231 cells 10 weeks after orthotopic transplantation (Figures 4d and e). However, such an event was never observed after transplantation of PKCλ/ι-depleted MDA-MB-231 cells (Figures 4d and e). As, the primary tumor growth after orthotopic transplantation was itself inhibited upon PKCλ/ι depletion, the lack of metastatic lung colonization of PKCλ/ι-depleted MDA-MB-231 cells could be a secondary effect due to lack of primary tumor growth. Therefore, to further confirm the functional importance of PKCλ/ι during breast cancer metastasis, we transplanted both control and PKCλ/ι-depleted MDA-MB-231-luc cells intravenously via tail vein (n=5) and monitored for metastatic lung colonization via BLI (Figure 4f). We observed significant inhibition in lung colonization with PKCλ/ι-depleted cells within 3 weeks (Figures 4g–i and Supplementary Figure S5). Collectively, our functional analyses in animal models strongly indicate that PKCλ/ι signaling is important to promote TNBC growth, invasion and metastasis in vivo.
Depletion of PKCλ/ι inhibits breast cancer metastasis. (a) Schematic of spontaneous metastasis assay using PKCλ/ι-depleted MDA-MB-231 cells (n=5). H&E and Ki67 staining (b) and quantification of Ki67 staining (n=18) (c) of breast tissues transplanted with MDA-MB-231 cells with or without PKCλ/ι depletion at 5 weeks after orthotopic transplantation. Results represent means±S.E.M. P-values were calculated using one-way ANOVA with Bonferroni post-test. H&E and Ki67 staining (d) and quantification of Ki67 staining (n=18) (e) of lung tissues at 10 weeks after orthotopic transplantation of MDA-MB-231 cells with or without PKCλ/ι depletion. Indicated region by perforated red lines showed metastatic growth at lung. Results represent means±S.E.M. P-values were calculated one-way ANOVA with Bonferroni post-test. (f) Schematic of metastatic lung colonization assay after intravenous transplantation of MDA-MB-231-luc cells with or without PKCλ/ι depletion. (g) Quantification of metastatic lung colonization via luminescence measurements (n=5). Results represent means±S.E.M. P-values were calculated using one-way ANOVA with Bonferroni post-test (see also Supplementary Figure S5 for detailed statistical analysis) (h) Representative whole-animal images at 3 weeks post-intravenous transplantation. (i) H&E staining of lung tissues 3 weeks after intravenous transplantation. Indicated regions by perforated red lines and arrows showed lung colonization
PKCλ/ι-regulated signature genes are associated with breast cancer invasive progression and poor clinical outcome
Although PKCλ/ι is highly expressed in IDC and metastatic breast cancers (Figure 1), we were unable to significantly correlate the PKCλ/ι mRNA expression data with clinicopathological parameters (Supplementary Figure S1a). These results suggest that active, phosphorylated-PKCλ/ι forms (significantly high in IDC and metastatic breast cancers as shown in Figure 1) might be functionally more significant during breast cancer progression. Hence, we hypothesized that alteration of gene expression owing to PKCλ/ι signaling could be an indicative parameter to assess clinical outcome in breast cancer patients. To test this hypothesis, we investigated global gene expression changes in PKCλ/ι−depleted MDA-MB-231 cells via RNA sequencing (Figure 5a and Supplementary Table S1). We found that 2367 genes were downregulated and 1537 genes were upregulated upon PKCλ/ι depletion. Out of these PKCλ/ι-regulated genes, we selected 2234 genes, whose expressions were significantly altered in MDA-MB-231 cells after PKCλ/ι depletion and to be regulated by known transcription factors (via IPA resources www.ingenuity.com; Supplementary Table S2). We evaluated expressions of those genes against publicly available breast cancer patient microarray data sets. We refined these genes to a set of 110 genes by a factor analysis-based filtering procedure (see details in Methods section) performed on a human breast cancer patient cohort of 31 DCIS, 36 IDC and 6 normal breast samples (Gene Expression Omnibus under accession GSE 26304).42 The set of 110 signature genes (Supplementary Table S3) was subsequently termed the PKCλ/ι invasive signature 110 (PKCλ/ιIS110), and their expression patterns stratified patients into three distinct clusters; normal breast (group 1), DCIS (group2) and IDC (group3) with cluster independence P-value of 0.0108 and empirical P-value of 0.0026 based on Rand Index (Figures 5b and c). Notably, a few basal-like (triple negative) DCIS cases clustered very closely with IDC groups indicating the similarity in molecular signatures between basal-like DCIS and IDC as reported earlier.42 We observed that, among PKCλ/ιIS110, 45 genes are favorably overexpressed in the normal breast group (NB specific), 3 genes are specifically overexpressed in the DCIS group (DCIS-specific) and 62 genes are overexpressed in the IDC group (IDC specific) (Figures 5b and c).
PKCλ/ι-regulated genes are differentially expressed in the normal breast, DCIS and IDC patients and associated with poor clinical outcome. (a) Schematic representation of global transcriptome changes in MDA-MB-231 cells with and without PKCλ/ι depletion as determined by RNA sequencing. (b) Heatmap representing the unsupervised k-means clustering of human patient data set GSE 26304 (n=73) using PKCλ/ιIS110 feature vector. Patient samples are distinctly grouped according to their disease type. The color gradient of the heatmap represents normalized expression values from high (red) to low (blue). A set of 62 genes specifically overexpressed in group 3 or IDC cluster (indicated as IDC specific, green bar along left side), 45 genes overexpressed in group 1 or normal breast cluster (indicated as NB Speci, yellow bar along left side), and three genes overexpressed in group 2 or DCIS cluster. One basal-like (triple negative) DCIS patient clustered in Group 3 indicated by black arrow. (c) Mean expression and expression polarization P-values of PKCλ/ιIS110 genes between patient groups of data set GSE 26304. (d) Heatmap representing the unsupervised k-means clustering of an independent human patient data set GSE3893-GPL570 containing matched DCIS and IDCs (n=14) using PKCλ/ιIS110 feature vector. While these samples show a natural tendency to cluster by patient, k-means clustering with PKCλ/ιIS110 feature vector distinctly groups them according to their disease type. The color gradient of the heatmap represents normalized expression values from high (red) to low (blue). All DCIS patients clustered in Group 1 (purple bar along the top), and six out of seven IDC patients clustered in Group 2 (red bar) indicating the validity of PKCλ/ιIS110 signature gene set to predict invasive phenotype of breast cancer. (e) Mean expression and expression polarization P-values of PKCλ/ιIS110 genes between patient groups of data set GSE3893-GPL570. (f) Heatmap representing unsupervised k-means (k=3) clustering in human patient data set GSE1456 (n=159) using PKCλ/ιIS110 feature vector. The color gradient of the heatmap represents normalized expression values from high (red) to low (blue). Group 1 (blue bar along the top), Group 2 (purple bar) and Group 3 (red bar). (g) Mean expression and expression polarization P-values of PKCλ/ιIS110 genes between patient groups of data set GSE1456. (h) Kaplan–Meier estimates of the time-to-relapse of patient samples (from GSE1456 cohort) of groups 2 (purple) and 3 (red) were compared with group 1 (blue). The P-values were calculated using the log-rank test. Patient group 1 (blue) showed better prognosis compared with groups 2 and 3. (i) Kaplan–Meier estimates of time-to-death due to breast cancer of patient samples (from GSE1456 cohort) of groups 2 (purple) and 3 (red) were compared with group 1(blue). The P-values were calculated using the log-rank test. Patient group1 (blue) showed better prognosis compared with groups 2 and 3
Next, we performed validation analysis with another independent human breast cancer patient cohort of seven DCIS and seven matched IDC samples (GSE3893-GPL570).43 Although these matched samples have inherent tendency to cluster together, we observed that PKCλ/ιIS110 stratified almost all IDC patients (six out of seven) in one group and all DCIS patients into another distinct group (cluster independence P-value 0.0031 and empirical P-value based on Rand Index is 0.0031) (Figures 5d and e). These data strongly indicate that altered expressions of PKCλ/ιIS110 genes could contribute to the invasive progression of breast cancer and PKCλ/ιIS110 gene set might serve as potential biomarkers to predict the DCIS to invasive breast cancer transition.
Consequently, we investigated whether downregulation of normal breast-specific genes or upregulation of IDC-specific genes of PKCλ/ιIS110 gene set could predict clinical outcome such as relapse or death in human breast cancer. We analyzed clinical relevance of the PKCλ/ιIS110 genes in a cohort of 159 human primary tumor samples representing breast cancer heterogeneity (25 basal-like or TNBC, 15 HER2-positive, 39 luminal-A, 23 luminal-B, 37 normal breast-like and 20 unclassified).44 The PKCλ/ιIS110 genes showed clinically relevant clustering of these patients into three subgroups independent of the breast cancer molecular subtypes (Figure 5f). Importantly, patients with loss of NB-specific genes and higher expression of IDC-specific genes predominantly clustered into the groups with poor clinical outcome (Group 2 and 3) (Figure 5g–i). Similar observations were also observed in another independent data set of 286 lymph-node-negative patients with known ER status (GSE 2034).45 The PKCλ/ιIS110 genes clustered patients into subgroups with significantly different clinical outcomes independent of ER status (Supplementary Figure S6), and, again, loss of normal breast-specific genes or higher expression of IDC-specific genes indicated poor outcome (Supplementary Figures S6b and c). Thus, our RNA-seq and multi-step bioinformatic analyses with gene expression data sets of human breast cancer patients have identified a PKCλ/ι-regulated gene signature predictive of invasive progression of human breast cancer and poor clinical outcome.
Cytokine-PKCλ/ι-RelA regulatory axis positively modulates progression of breast cancer
As our analysis in microarray data sets of human breast cancer patients indicated clinical significance of differential expressions of PKCλ/ιIS110 genes, we performed bio-function pathway analysis via ingenuity resources (IPA). We found that the expression pattern of PKCλ/ιIS110 genes in MDA-MB-231 cells indicated significant inhibition of various cellular pathways involved in cancer progression such as cell movement, migration, invasion and metastasis (Supplementary Figure S7a). Consequently, we wanted to understand potential signaling cascades regulating PKCλ/ιIS110 genes via activation of PKCλ/ι. Our analysis for upstream regulators potentially associated with the regulation of PKCλ/ιIS110 genes (via IPA) indicated that a majority of the PKCλ/ιIS110 genes could be regulated through various cytokines including TGFβ, IL1β and TNFα (Supplementary Table S4 and Supplementary Figure S7b). All these cytokines have been implicated in breast cancer progression previously.46, 47, 48 To test the effect of these cytokines on PKCλ/ι activation, we treated MDA-MB-231 cells with different cytokines and tested phosphorylation of PKCλ/ι. Intriguingly, we found that both TGFβ1 and IL1β, individually and in combination, induced PKCλ/ι phosphorylation in MDA-MB-231 cells (Figure 6a and Supplementary Figure S8a). However, we did not observe induction of PKCλ/ι phosphorylation upon TNFα treatment (Supplementary Figure S8a). Interestingly, we also observed that cytokine-induced phospho-PKCλ/ι molecules are predominantly localized within the nuclei of MDA-MB-231 cells (Figure 6b). Taken together, these data strongly indicate that cytokines, like TGFβ and IL1β, might induce PKCλ/ι activation in TNBC cells and facilitate nuclear translocation of phospho-PKCλ/ι as observed in our xenogtraft models as well as in IDC and metastatic breast cancers (Figure 1 and Figure 3g).
Involvement of Cytokine-PKCλ/ι-NF-κB regulatory axis in TNBC cells. (a) Phosphorylation of PKCλ/ι in MDA-MB-231 cells was significantly induced after TGFβI and IL1β treatment. For quantification, results represent means±S.E.M. (n=3), and P-value was calculated using one-way ANOVA with Bonferroni post-test. (b) Induction of phospho-PKCλ/ι predominantly noticed in the nucleus. For quantification, results represent means±S.E.M. (n=3) and P-value was calculated using two-tailed unpaired Student's t test. (c) Localization of NF-κBp65 (RelA) in MDA-MB-231 cells with and without PKCλ/ι depletion. RelA expression showed in red and nuclear staining showed by DAPI. Arrows indicated nuclear localization of RelA. (d) Western blot analysis indicating no significant changes in RelA expression level after PKCλ/ι depletion, rather, impaired RelA nuclear translocation. For quantification, results represent means±S.E.M. (n=3) and P-value was calculated using one-way ANOVA with Bonferroni post-test. (e) NF-κB reporter gene assay of MDA-MB-231 cells with and without PKCλ/ι depletion (n=3). Results represent means±S.E.M. P-values were calculated using two-tailed unpaired Student’s t-test. (f) Quantitative RT-PCR measurements of RelA target genes DAB2, VIM, ICAM1 and PLAU in MDA-MB-231 cells with and without PKCλ/ι depletion (n=3). Results represent means±S.E.M. (g) Quantitative PCR-based measurements of RelA chromatin occupancy at the promoter regions of DAB2, VIM, ICAM1 and PLAU genes in MDA-MB-231 cells with and without PKCλ/ι depletion (n=3). Results represent means±S.E.M. (h) Schematic of cytokine signaling-PKCλ/ι-NF-κB p65 regulatory axis for breast cancer growth, invasion and metastasis
To link different cytokine signaling nodes and PKCλ/ι, we looked for a common downstream regulator. Interestingly, earlier reports implicated atypical PKC signaling in regulating NF-κB p65 (RelA) function in multiple cell types,49, 50 and RelA has a known association with various cytokine networks51, 52 (Supplementary Figure S7b). As RelA is one of the major transcription factors that inhibited upon PKCλ/ι depletion in MDA-MB-231 cells (as predicted by IPA in Supplementary Table S3), we tested RelA expression in multiple TNBC cells along with HER2-positive BT474 and ER+ MCF-7 cells. We found that RelA was highly abundant in all these cell types (Supplementary Figure S8b). Thus, we hypothesized that a PKCλ/ι-RelA signaling axis might regulate the expression of at least some of the PKCλ/ιIS110 genes during breast cancer disease progression. For confirmation, we tested RelA nuclear localization and activity in MDA-MB-231 cells after PKCλ/ι depletion. We observed significant reduction of RelA nuclear localization in MDA-MB-231 cells after PKCλ/ι depletion (Figures 6c, 6d and Supplementary Figure S8c). We confirmed no significant changes in the total RelA expression level in MDA-MB-231 cells after PKCλ/ι depletion (Figure 6d). To validate compromised RelA function, we performed NF-κB reporter gene analysis and observed significant repression of NF-κB transcription activity in the PKCλ/ι-depleted MDA-MB-231 cells (Figure 6e). For further confirmation, we measured mRNA expression levels of known RelA target genes from PKCλ/ιIS110 signature panel such as ICAM1 (intercellular adhesion molecule 1)53 and PLAU (plasminogen activator, Urokinase)54 and also the genes that contain consensus RelA-binding motifs at the promoter regions such as DAB2 (Dab, mitogen-responsive phosphoprotein, homolog 2 (Drosophila)) and VIM (Vimentin) (Supplementary Figure S8d). We observed significant downregulation of these genes in PKCλ/ι-depleted cells compared with control (Figure 6f). Furthermore, RelA chromatin occupancy at the consensus RelA-binding motifs present in the promoter regions of these genes was also impaired (Figure 6g).
To confirm further that RelA transcriptional activity downstream to PKCλ/ι signaling might contribute to gene regulation and invasiveness of TNBC cells, we ectopically expressed a constitutively active RelA mutant (RelA-S536E)55 in PKCλ/ι-depleted MDA-MB-231 cells (Supplementary Figure S9). We observed that ectopic expression of RelA-S536E partially rescues expression of RelA target genes in PKCλ/ι-depleted MDA-MB-231 cells and promotes their invasiveness when tested via in vitro invasion assay (Supplementary Figure S9). These results strongly indicate that a cytokine-PKCλ/ι-RelA signaling axis might be involved in gene regulation to positively modulate invasive and metastatic progression of breast cancer cells (Figure 6h).
Discussion
In this study, we provided evidence that PKCλ/ι signaling positively regulates TNBC invasion and metastasis. Specific depletion of PKCλ/ι inhibited invasiveness of multiple TNBC breast cancer cell types with mutant TP53, EGFR overexpression and BRCA1 mutation. All these genetic abnormalities are significantly associated with poor prognosis.8, 56 Importantly, depletion of PKCλ/ι significantly inhibited TNBC growth and metastatic lung colonization in experimental animal models. These observations indicate that targeting PKCλ/ι signaling might have therapeutic potential for TNBC relapse and metastasis.
During breast cancer progression, transitions from normal breast to DCIS and then to IDC are associated with important developmental changes. However, to date, the molecular basis of these developmental changes is poorly understood. Intriguingly, we observed a gradual increase in PKCλ/ι signaling from normal breast to DCIS to IDC samples of multiple clinical subtypes, indicating that gradual increase in PKCλ/ι activity might be important for altered gene expression circuitry associated with breast cancer progression. Consistently, our global gene expression analysis in breast cancer cells and human breast cancer patient data sets identified a PKCλ/ι dependent gene signature (PKCλ/ιIS110) differentially expressed between normal breast, DCIS and IDC samples. We also observed that differential expression of IDC-specific genes of PKCλ/ιIS110 is significantly associated with poor clinical outcome such as relapse and death of breast cancer patients. Thus, PKCλ/ιIS110 could be of tremendous clinical significance and might serve as a platform to identify specific biomarkers, which could predict invasive progression of breast cancer. Altogether, these observations and previous reports28, 29 indicate significance of PKCλ/ι signaling during the breast cancer disease progression including growth, invasion, relapse/metastasis and survival.
Mechanistically, we have identified cytokine-PKCλ/ι-NF-κB signaling axis, which could regulate key genes associated with breast cancer progression and metastasis. IPA analysis identified that depletion of PKCλ/ι resulted in inhibition of multiple cytokine signaling nodes such as TGFβ1 and IL-1β; both cytokines are known to control expressions of various migration- and metastasis-related genes.57, 58 Supporting IPA analysis, we found that TGFβ1 and IL-1β could induce phosphorylation as well as nuclear translocation of PKCλ/ι in TNBC cells. Previously, various lipids and growth factors, such as ceramide, phosphoinositide 3-kinase (PI3K), 3 phosphoinositide-dependent protein kinase-1 (PDK-1), EGF, NGF, PDGF and HER2, have been implicated as the upstream regulators to activate PKCλ/ι.15, 31 Thus, it is possible that TGFβ1 and IL-1β could modulate these known activators of PKCλ/ι to induce phosphorylation at Thr555/Thr563. However, further research is required to investigate specific molecular mechanism.
We also observed that NF-κB p65 or RelA transcriptional activity in TNBC cells could be regulated by PKCλ/ι signaling. Furthermore, rescue of invasive phenotype of PKCλ/ι-depleted MDA-MB-231 cells upon ectopic expression of constitutively active RelA-S536E molecules strongly indicates that a PKCλ/ι-RelA signaling axis, at least in part, might contribute to the invasive progression of TNBC cells. However, our global gene expression analysis also indicated that depletion of PKCλ/ι signaling could repress additional transcriptional networks, such as hypoxia-inducible factor 1alpha (HIF1α), signal transducer and activator of transcriptions (STATs) and SMAD3, known to function downstream of various cytokine and growth factor signaling networks to promote breast cancer invasion and metastasis.14 These observations imply the existence of overlapping regulatory mechanisms involving PKCλ/ι and other signaling cascades to promote invasive progression of breast cancer, and future studies in this direction could provide important information.
In summary, we discovered that PKCλ/ι signaling mediates survival adaptability of breast cancer cells in their natural milieu, that is, in the breast, and promotes metastasis in distal tissues such as the lung by regulating NF-κB and other cytokine and growth factor-associated transcription factors. Thus, our study raised a possibility to treat highly heterogeneous breast cancer disease by targeting PKCλ/ι signaling and its upstream and downstream regulatory molecules.
Materials and Methods
Analysis of human primary breast tumor samples
The tissue microarray slides consisting of 55 IDC samples (10 ER positive, 10 HER2 positive, 35 TNBC), 10 DCIS samples and 10 normal breast tissues were prepared by the University of Kansas Medical Center Department of Pathology from archival material following IRB approval. Expressions of both PKCλ/ι and phospho-PKCλ/ι were analyzed by immunohistochemistry (IHC). Digital images of the stained slides were taken using Aperio TMA software, and expressions of PKCλ/ι and phospho-PKCλ/ι were analyzed by two independent pathologists in a double-blind manner. The expression levels (IHC scores) were indicated in a scale of 0 to 3, where 3 indicates highest and 0 indicates lowest expressions. IHC scores 0 to 1 and >1 were considered as low and high expression, respectively. For significance, P-values were calculated by two-way ANOVA with Boneferroni post-test (Supplementary Figures S1c–f).
Cell lines
All breast cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained according to the recommended protocol. For knockdown of PKCλ/ι, cell lines were infected with lentiviral pGIPZ shRNAmir vector containing short hairpins and GFP reporter (Supplementary Table S5) (Open Biosystems, Pittsburgh, PA, USA).
Animal studies
All animal works were done in accordance with approved protocol by the Institutional Animal Care and Use Committee. Female NU/NU nude and NOD-SCID NSG mice (Charles River) of 4–6 weeks old (n=5 for cell line for each in vivo experimental set) were used in xenograft studies for lung metastasis and orthotopic mammary fat pad tumor assays. Two different shRNA constructs showed most effective knockdown of PKCλ/ι and subsequently used to knockdown PKCλ/ι in TNBC cells including MDA-MB-231-luc or MDA-MB-231 cells that were used for animal studies.
For orthotopic tumor assays, cells were collected in PBS. Mice were anesthetized, a small incision was made to reveal the mammary gland and 2 × 106 cells were injected directly into the second or fourth mammary glands. The incision was closed with wound clips, and primary tumor outgrowth was monitored weekly. For spontaneous metastasis assays, primary tumors were surgically removed after 5 weeks and kept for another 5 weeks for the development of metastasis in the lungs.
For lung colonization assay, 1 × 106 viable cells were injected into the lateral tail vein in a volume of 0.1 ml. Following isoflurane-induced anesthesia, mice were imaged for luciferase activity immediately after injection to exclude any that were not successfully xenografted. Lung colonization was monitored weekly and continued up to 3 weeks.
Bioluminescent imaging
Mice were anesthetized and injected intraperitoneally (IP) with D-luciferin (15 mg/ml in PBS and 0.01 ml/g bodyweight). Imaging was performed after injection with a Xenogen IVIS system coupled to Living Image acquisition and analysis software version 4.0 (Xenogen, Hopkinton, MA, USA). Region of interest (ROI) boxes were drawn around the entire body (excluding tail) of the animals. Measurements were expressed as flux (i.e., photons/second) and scaled to a comparable background value (from a luciferin-injected mouse with no tumor cells). For normalization, the flux values obtained immediately after xenografting (day 0) were considered as 100 so that all mice had an arbitrary starting BLI signal of 100.
RNA-Seq analysis and Gene set Enrichment Analysis
We used whole-transcriptome shotgun sequencing (RNA-Seq) to obtained the global gene expression profile of MDA-MB-231 cells after PKCλ/ι depletion by RNAi carried out independently using two different shRNA clones (shRNA1 and shRNA2, Supplementary Table S5) and compared it with the expression profile under control conditions (control shRNA, Supplementary Table S5). Detailed methods were described in Supplementary Materials and Methods.
Patient data set analysis
All genes were normalized to the geometric average of the three housekeeping genes MRPL19, PSMC4 and PUM1, as these genes showed minimal variation in expression across a variety of experimental conditions.59 Genes with multiple probesets were represented by the probeset with maximal variance across samples. The significance of the partitions generated by k-means clustering for the 110 gene feature vector (PKCλ/ιIS110) was evaluated as an empirical P-value calculated on the χ2-statistic of cluster independence as follows: PChi=(c+1)/(N+1), where c is the number of clusterings for randomly selected genes of the same size as the signature gene set that generated a χ2-statistic equal to or greater than the observed χ2-statistic and N (10,000) the number of random tests performed. The empirical P-value based on the Rand index was calculated similarly giving an alternate assessment of the significance of the generated clusters. Here PRand=(r+1)/(N+1) where r is the number of clusterings for randomly selected genes of the same size as the signature gene set that generated a Rand measure equal to or greater than the observed Rand measure and N (10,000) the number of random tests performed. Population survival or remission curves were compared using the log-rank test. The survival function was estimated using the Kaplan–Meier procedure. Gene expression levels were standardized (mean 0 and S.D. 1) across samples for visualization. Expression polarization was defined as the absolute difference between the mean expression levels over the genes and patients in each group, and P-values of the observed expression polarization was calculated as described earlier.60
Among the 3904 significantly differentially expressed genes in the RNA-Seq experiments, 2234 genes were identified that were associated with significantly inhibited or activated upstream regulators as predicted by IPA. With these genes as observations and samples as variables, the data set GSE26304 was factor analyzed with three common factors. The factor solution was rotated using the ‘varimax’ procedure for better interpretation. Genes with an absolute correlation coefficient ≥0.6 with the factor loadings of any one of the factors were selected to comprise the signature set of 110 genes (PKCλ/ιIS110). These 110 signature genes formed the feature vector for further k-means clustering on the data sets GSE26304, GSE3893-GPL570, GSE1456 and GSE 2034. Kaplan–Meier estimation was performed using time-to-death due to breast cancer or censoring and time-to-relapse or last follow-up for GSE1456 and GSE 2034 data sets. Genes from the data set GSE26304 were hierarchically clustered using the Euclidian pair-wise distance between rows and the ‘ward’ method for the linkage function to establish the signature gene order.
Statistics
All statistical analyses were performed using GraphPad Prism5 statistical software (GraphPad Software Inc., San Diego, CA, USA) unless mentioned otherwise. All data are expressed as means±S.E.M. unless mentioned otherwise. P-values were calculated by two-tailed unpaired Student's t-test, one-way or two-way ANOVA with Bonferroni post-test unless mentioned otherwise, and P<0.05 was considered as significant.
Accession codes
Abbreviations
- AJC:
-
apical-junctional complex
- ADH:
-
atypical ductal hyperplasia
- BLI:
-
bioluminescence imaging
- BRCA1:
-
breast cancer 1, early onset
- DAB2:
-
Dab, mitogen-responsive phosphoprotein, homolog 2
- DCIS:
-
ductal carcinoma in situ
- EGFR:
-
epidermal growth factor receptor
- ER:
-
estrogen receptor
- HER2:
-
epidermal growth factor receptor 2
- HIF1α:
-
hypoxia-inducible factor 1alpha
- ICAM1:
-
intercellular adhesion molecule 1
- IDC:
-
invasive ductal carcinoma
- IHC:
-
immunohistochemistry
- IL1β:
-
interleukin-1 beta
- MRPL19:
-
mitochondrial ribosomal protein L19
- NF-κB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAR3:
-
partitioning defective 3 homolog
- PAR6:
-
partitioning defective 6 Homolog
- PKCλ/ι:
-
atypical protein kinase C lamda/iota
- PKCζ:
-
atypical protein kinase C zeta
- PKCλ/ιIS110:
-
PKCλ/ι invasive signature 110
- PLAU:
-
plasminogen activator, urokinase
- PR:
-
progesterone receptor
- PSMC4:
-
proteasome (prosome, macropain) 26 S subunit, ATPase, 4
- PUM1:
-
pumilio RNA-binding family member 1
- RNA:
-
ribonucleic acid
- RNAi:
-
RNA interference
- SMAD3:
-
SMAD family member 3
- STAT:
-
signal transducer and activator of transcription
- TGFβ:
-
transforming growth factor beta
- TNBC:
-
triple-negative breast cancer
- TP53:
-
tumor Protein p53
- VIM:
-
vimentin
References
Stecklein SR, Jensen RA, Pal A . Genetic and epigenetic signatures of breast cancer subtypes. Front Biosci Elite, Ed 2012; 4: 934–949.
Foulkes WD, Smith IE, Reis-Filho JS . Triple-negative breast cancer. N Engl J Med 2010; 363: 1938–1948.
Peddi PF, Ellis MJ, Ma C . Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer 2012; 2012: 217185.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13 (15 Pt 1): 4429–4434.
Yagata H, Kajiura Y, Yamauchi H . Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer 2011; 18: 165–173.
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA . Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009; 115: 423–428.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874.
Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N . Triple-negative breast cancer—current status and future directions. Ann Oncol 2009; 20: 1913–1927.
Nofech-Mozes S, Trudeau M, Kahn HK, Dent R, Rawlinson E, Sun P et al. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Cancer Res Treat 2009; 118: 131–137.
Fields AP, Regala RP . Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacol Res 2007; 55: 487–497.
Urtreger AJ, Kazanietz MG . Bal de Kier Joffe ED. Contribution of individual PKC isoforms to breast cancer progression. IUBMB Life 2012; 64: 18–26.
Griner EM, Kazanietz MG . Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 2007; 7: 281–294.
Koivunen J, Aaltonen V, Peltonen J . Protein kinase C (PKC) family in cancer progression. Cancer Lett 2006; 235: 1–10.
McCaffrey LM, Montalbano J, Mihai C, Macara IG . Loss of the par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 2012; 22: 601–614.
Suzuki A, Akimoto K, Ohno S . Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J Biochem 2003; 133: 9–16.
Suzuki S, Moore DH 2nd, Ginzinger DG, Godfrey TE, Barclay J, Powell B et al. An approach to analysis of large-scale correlations between genome changes and clinical endpoints in ovarian cancer. Cancer Res 2000; 60: 5382–5385.
Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 12519–12524.
Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM et al. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 2005; 65: 8905–8911.
Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP . Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem 2005; 280: 31109–31115.
Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR . Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res 2010; 70: 2064–2074.
Takagawa R, Akimoto K, Ichikawa Y, Akiyama H, Kojima Y, Ishiguro H et al. High expression of atypical protein kinase C lambda/iota in gastric cancer as a prognostic factor for recurrence. Ann Surg Oncol 2010; 17: 81–88.
Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D et al. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol 2004; 164: 797–802.
Yang YL, Chu JY, Luo ML, Wu YP, Zhang Y, Feng YB et al. Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosomes Cancer 2008; 47: 127–136.
Du GS, Wang JM, Lu JX, Li Q, Ma CQ, Du JT et al. Expression of P-aPKC-iota, E-cadherin, and beta-catenin related to invasion and metastasis in hepatocellular carcinoma. Ann Surg Oncol 2009; 16: 1578–1586.
Li Q, Wang JM, Liu C, Xiao BL, Lu JX, Zou SQ . Correlation of aPKC-iota and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2008; 7: 70–75.
Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T, Ishiguro-Imagawa Y et al. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci USA 2009; 106: 16369–16374.
Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo-Duncan M . Involvement of PKC-iota in glioma proliferation. Cell Prolif 2008; 41: 122–135.
Kojima Y, Akimoto K, Nagashima Y, Ishiguro H, Shirai S, Chishima T et al. The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum Pathol 2008; 39: 824–831.
Paget JA, Restall IJ, Daneshmand M, Mersereau JA, Simard MA, Parolin DA et al. Repression of cancer cell senescence by PKCiota. Oncogene 2012; 31: 3584–3596.
Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T et al. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 1998; 143: 95–106.
Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 2006; 8: 1235–1245.
Joberty G, Petersen C, Gao L, Macara IG . The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2000; 2: 531–539.
Ishiuchi T, Takeichi M . Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol 2011; 13: 860–866.
Wald FA, Forteza R, Diwadkar-Watkins R, Mashukova A, Duncan R, Abreu MT et al. Aberrant expression of the polarity complex atypical PKC and non-muscle myosin IIA in active and inactive inflammatory bowel disease. Virchows Arch 2011; 459: 331–338.
Kikuchi K, Soundararajan A, Zarzabal LA, Weems CR, Nelon LD, Hampton ST et al. Protein kinase C iota as a therapeutic target in alveolar rhabdomyosarcoma. Oncogene 2013; 32: 286–295.
He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009; 137: 635–646.
Messerschmidt A, Macieira S, Velarde M, Badeker M, Benda C, Jestel A et al. Crystal structure of the catalytic domain of human atypical protein kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol 2005; 352: 918–931.
Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJ . Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci 2008; 121 (Pt 5): 644–654.
Avila MA, Velasco JA, Cansado J, Notario V . Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res 1994; 54: 2424–2428.
Castles CG, Fuqua SA, Klotz DM, Hill SM . Expression of a constitutively active estrogen receptor variant in the estrogen receptor-negative BT-20 human breast cancer cell line. Cancer Res 1993; 53: 5934–5939.
Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, Tonk V et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res 1998; 58: 3237–3242.
Muggerud AA, Hallett M, Johnsen H, Kleivi K, Zhou W, Tahmasebpoor S et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol 2010; 4: 357–368.
Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M et al. Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res 2006; 66: 5278–5286.
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 2005; 7: R953–R964.
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005; 365: 671–679.
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013; 15: 807–817.
Bierie B, Moses HL . Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006; 6: 506–520.
Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T et al. Inflammatory mediators in breast cancer: coordinated expression of TNFalpha & IL-1beta with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011; 11: 130.
Dutta D, Ray S, Home P, Larson M, Wolfe MW, Paul S . Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance. Stem Cells 2011; 29: 618–628.
Duran A, Diaz-Meco MT, Moscat J . Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J 2003; 22: 3910–3918.
Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM . NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol 2006; 176: 603–615.
Martone R, Euskirchen G, Bertone P, Hartman S, Royce TE, Luscombe NM et al. Distribution of NF-kappaB-binding sites across human chromosome 22. Proc Natl Acad Sci USA 2003; 100: 12247–12252.
Anrather J, Racchumi G, Iadecola C . cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-kappa B. J. Biol Chem 2005; 280: 244–252.
Wang W, Abbruzzese JL, Evans DB, Chiao PJ . Overexpression of urokinase-type plasminogen activator in pancreatic adenocarcinoma is regulated by constitutively activated RelA. Oncogene 1999; 18: 4554–4563.
Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L et al. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 2005; 25: 7966–7975.
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010; 28: 1145–1153.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008; 133: 66–77.
Hiratsuka S, Watanabe A, Aburatani H, Maru Y . Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006; 8: 1369–1375.
Szabo A, Perou CM, Karaca M, Perreard L, Palais R, Quackenbush JF et al. Statistical modeling for selecting housekeeper genes. Genome Biol 2004; 5: R59.
Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 2012; 18: 529–537.
Acknowledgements
We thank Dr Andrew L. Kung, Dana-Farber Cancer Institute for providing FUW-Luc-mCherry construct. The work is supported by NIH grants HD062546, HL094892, HD075233. PH is supported by a postdoctoral fellowship from American Heart Association. This work was also supported by a gift from Deffenbaugh Foundation and a grant from the Kansas Intellectual and Developmental Disabilities Research Center (HD002528) and Illumina to AP.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Paul, A., Gunewardena, S., Stecklein, S. et al. PKCλ/ι signaling promotes triple-negative breast cancer growth and metastasis. Cell Death Differ 21, 1469–1481 (2014). https://doi.org/10.1038/cdd.2014.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.62
This article is cited by
-
Macrophages-aPKCɩ-CCL5 Feedback Loop Modulates the Progression and Chemoresistance in Cholangiocarcinoma
Journal of Experimental & Clinical Cancer Research (2022)
-
Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells
BMC Cancer (2017)
-
PKCiota promotes ovarian tumor progression through deregulation of cyclin E
Oncogene (2016)
-
Involvement of Tight Junction Plaque Proteins in Cancer
Current Pathobiology Reports (2016)
-
PKCζ Promotes Breast Cancer Invasion by Regulating Expression of E-cadherin and Zonula Occludens-1 (ZO-1) via NFκB-p65
Scientific Reports (2015)