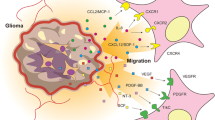
Abstract
The disseminated neoplastic foci of malignant gliomas are essentially responsible for the limited efficacy of current available therapeutic modalities. Bone marrow-derived stem cells (BMSCs) have the ability to migrate into these tumors and even track infiltrating tumor cells, making them to be promising cellular vehicles for delivering therapeutic agents to glioma cells. The herpes simplex virus thymidine kinase (HSV–TK)/ganciclovir (GCV) suicide gene therapy with a potent bystander effect has been considered as one of the most promising therapeutic strategies for malignant gliomas. In this study, we evaluate the anti-glioma effect of suicide gene therapy using BMSCs expressing HSV–TK combined with overexpression of connexin 43 (Cx43), which can restore the gap junction of intercellular communication and may enhance the bystander effect of suicide gene therapy. To assess the potential of BMSCs to track glioma cells, a spheroid co-culture system in matrigel was used to show that some BMSCs migrated to C6 glioma cell microspheres. Transwell assay showed the tumor tropic property of BMSCs. In addition, BrdU-labeled BMSCs injected directly into the cerebral hemisphere opposite to the established C6 rat gliomas were capable of migrating into the xenograft gliomas. C6 cell growth was more intensively inhibited by HSV–TK/GCV treatment mediated by BMSCs, and could be further enhanced by combination with Cx43 transfection into glioma cells. The same result was observed in vivo by the growth of C6 gliomas and the survival analysis of rats bearing C6 glioma. In conclusion, Cx43 combined with HSV–TK/GCV gene therapy using BMSCs as vehicles was highly effective in a rat glioma model and therefore hold great potential as a novel approach for the gene therapy of human malignant gliomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res 2002; 62: 7170–7174.
Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS . The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res 2002; 62: 5657–5663.
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J et al. Human bone marrowderived mesenchymal stem cells in the treatment of gliomas. Cancer Res 2005; 65: 3307–3318.
Bianco P, Riminucci M, Gronthos S, Robey PG . Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001; 19: 180–192.
Colter DC, Class R, DiGirolamo CM, Prockop DJ . Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 2000; 97: 3213–3218.
Koc ON, Lazarus HM . Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant 2001; 27: 235–239.
Vincent AJ, Vogels R, Someren GV, Esandi MC, Noteboom JL, Avezaat CJ et al. Herpes simplex virus thymidine kinase gene therapy for rat malignant brain tumors. Hum Gene Ther 1996; 7: 197–205.
Rosolen A, Frascella E, di Francesco C, Todesco A, Petrone M, Mehtali M et al. In vitro and in vivo antitumor effect of retrovirus-mediated herpes simplex thymidine kinase gene-transfer in human medulloblastoma. Gene Ther 1998; 5: 113–120.
Peister A, Mellad J, Larson B, Hall B, Gobson L, Prockop D . Adult stem cells from bone marrow (MSCs) isolated from different strains of in bred mice vary in surface epitopes, rates of proliferation, and differential potential. Blood 2004; 103: 1662–1668.
Wang SL, Li F, Pu PY, Wang GX, Peng Q, Wang CY . The establishment and characterization of human glioblastoma cell lines TJ899 and TJ905. Tianjin Med J 1996; 24: 416–418. (in Chinese).
Pu PY, Liu XW, Liu AX, Cui J, Zhang Y . Inhibitory effect of antisense epidermal growth factor receptor RNA on the proliferation of rat C6 glioma cells in vitro and in vivo. J. Neurosurg 2000; 92: 132–139.
Conget PA, Minguell JJ . Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 1999; 181: 67–73.
Heese O, Disko A, Zirkel D, Westphal M, Lamszus K . Migration of human neural stem cells toward an intracranial glioma. Neuro Oncol 2005; 7: 476–484.
Jeon JY, An JH, Kim SU, Park HG, Lee MA . Migration of human neural stem cells toward an intracranial glioma. Exp Mol Med 2008; 40: 84–91.
Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA 2000; 97: 12846–12851.
Touraine RL, Ishii-Morita H, Ramsey WJ, Blaese RM . The bystander effect in the HSVtk/ganciclovir system and its relation to gap junctional communication. Gene Ther 1998; 5: 1705–1711.
Touraine RL, Vahanian N, Ramsey WJ, Blaese RM . Enhancement of the herpes simplex virus thymidine kinase/ ganciclovir bystander effect and its antitumor efficacy in vivo by pharmacologic manipulation of gap junctions. Hum Gene Ther 1998; 9: 2385–2391.
Bi WL, Parysek LM, Warnick R, Stambrook PJ . In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV-TK retroviral gene therapy. Hum Gene Ther 1993; 4: 725–731.
Immonen A, Vapalahti M, Tyynel K, Hurskainen H, Sandmair A, Vanninen R et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 2004; 10: 967–972.
Rainov NGA . Phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 2000; 11: 2389–2401.
Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther 2000; 1: 195–203.
Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H . Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther 2005; 12: 600–607.
Uhl M, Weiler M, Wick W, Jacobs AH, Weller M, Herrlinger U . Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem Biophys Res Commun 2005; 328: 125–129.
Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther 2007; 15: 1373–1381.
Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 2000; 6: 447–450.
Staflin K, Honeth G, Kalliomaki S, Kjellman C, Edvardsen K, Lindvall M . Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res 2004; 64: 5347–5354.
Pu PY, Xia ZB, Huang Q, Wang CY, Wang GX . The effect of transfected Cx43 gene on the GJIC and proliferation of glioma cells. Chin J Cancer Res 2002; 14: 100–104. (in English).
Xia ZB, Pu PY, Huang Q, Zhang YT, Jiang WY, You YP . Connexin 43 gene for the treatment of rat C6 cerebral gliomas in vivo. Chin J Oncol 2002; 24: 212–214. (in Chinese).
Huang RP, Fan Y, Mohammad Z, Peng A, Zeng ZL, Boynton AL . Reversion of the neoplastic phenotype of human glioblastoma cells by Connexin43 (Cx43). Cancer Res 1998; 58: 5089–5096.
Naus CC, Elisevich K, Zhu D, Belliveau DJ, Del Maestro RF . In vivo growth of C6 glioma cells transfected with connexin43 cDNA. Cancer Res 1992; 52: 4208–4213.
Chen SC, Pelletier DB, Ao P, Boynton Al . Connexin 43 reverses the phenotype of transformed cells and alters their expression of cyclin/cyclin-dependent kinases. Cell Growth Differ 1995; 6: 681–690.
Pu P, Xia Z, Yu S, Huang Q . Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg 2004; 107: 49–54.
Shinoura N, Chen L, Wani MA, Kim YG, Larson JJ, Warnick RE et al. Protein and messenger RNA expression of connexin 43 in astrocytomas: implications in brain tumor gene therapy. J Neurosurg 1996; 84: 839–846.
Huang RP, Hossain MZ, Sehgal A . Reduced connexin 43 expression in high grade human brain glioma cells. J Surg Oncol 1999; 70: 21–24.
Soroceanu L, Manning Jr TJ, Sontheimer H . Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 2001; 33: 107–117.
Acknowledgements
This work was supported by grants from Tianjin Education Committee (Grant No. 20050227) and Tianjin Science and Technology Committee (Grant No. 09JCZDJC20500).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, Q., Liu, XZ., Kang, CS. et al. The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer Gene Ther 17, 192–202 (2010). https://doi.org/10.1038/cgt.2009.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.64
Keywords
This article is cited by
-
In vitro and in vivo double-enhanced suicide gene therapy mediated by generation 5 polyamidoamine dendrimers for PC-3 cell line
World Journal of Surgical Oncology (2012)
-
The Role of Gap Junction Channels During Physiologic and Pathologic Conditions of the Human Central Nervous System
Journal of Neuroimmune Pharmacology (2012)