Abstract
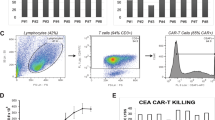
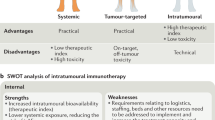
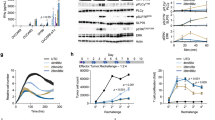
Metastatic spread of colorectal cancer (CRC) to the peritoneal cavity is common and difficult to treat, with many patients dying from malignant bowel obstruction. Chimeric antigen receptor T cell (CAR-T) immunotherapy has shown great promise, and we previously reported murine and phase I clinical studies on regional intrahepatic CAR-T infusion for CRC liver metastases. We are now studying intraperitoneal (IP) delivery of CAR-Ts for peritoneal carcinomatosis. Regional IP infusion of CAR-T resulted in superior protection against carcinoembryonic antigen (CEA+) peritoneal tumors, when compared with systemically infused CAR-Ts. IP CAR-Ts also provided prolonged protection against IP tumor re-challenges and demonstrated an increase in effector memory phenotype over time. IP CAR-Ts provided protection against tumor growth at distant subcutaneous (SC) sites in association with increases in serum IFNγ levels. Given the challenges posed by immunoinhibitory pathways in solid tumors, we combined IP CAR-T treatment with suppressor cell targeting. High frequencies of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) were found within the IP tumors, with MDSC expressing high levels of immunosuppressive PD-L1. Combinatorial IP CAR-T treatment with depleting antibodies against MDSC and Treg further improved efficacy against peritoneal metastases. Our data support further development of combinatorial IP CAR-T immunotherapy for peritoneal malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C et al. Peritoneal carcinomatosis. World J Gastroenterol 2013; 19: 6979–6994.
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H . 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008; 15: 2426–2432.
Cao C, Yan TD, Black D, Morris DL . A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009; 16: 2152–2165.
Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res 2015; 21: 3149–3159.
Saied A, Licata L, Burga RA, Thorn M, McCormack E, Stainken BF et al. Neutrophil:lymphocyte ratios and serum cytokine changes after hepatic artery chimeric antigen receptor-modified T-cell infusions for liver metastases. Cancer Gene Ther 2014; 21: 457–462.
Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Imunol Immunother 2015; 64: 817–829.
Rosenberg SA, Restifo NP . Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348: 62–68.
Saied A, Pillarisetty VG, Katz SC . Immunotherapy for solid tumors–a review for surgeons. J Surg Res 2014; 187: 525–535.
Lo AS, Ma Q, Liu DL, Junghans RP . Anti-GD3 chimeric sFv-CD28/TCRzeta designer T cells for treatment of melanoma and other neuroectodermal tumors. Clin Cancer Res 2010; 16: 2769–2780.
Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK . Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 2006; 20: 1819–1828.
Eshhar Z . Adoptive cancer immunotherapy using genetically engineered designer T-cells: First steps into the clinic. Curr Opin Mol Ther 2010; 12: 55–63.
Becker JC, Andersen MH, Schrama D, Thor Straten P . Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother 2013; 62: 1137–1148.
Kershaw MH, Westwood JA, Darcy PK . Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13: 525–541.
Thorn M, Point GR, Burga RA, Nguyen CT, Joseph Espat N, Katz SC . Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1+CD11b+ myeloid cells. J Leukoc Biol 2014; 96: 883–894.
Lee JC, Hayman E, Pegram HJ, Santos E, Heller G, Sadelain M et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res 2011; 71: 2871–2881.
Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014; 6: 261ra151.
Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA . Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005; 12: 65–71.
Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 2015; 3: 610–619.
Blank C, Mackensen A . Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56: 739–745.
John LB, Devaud C, Duong CM, Yong C, Beavis PA, Haynes NM et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013; 19: 5636–5646.
Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J . PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009; 21: 1065–1077.
Khaled YS, Ammori BJ, Elkord E . Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol 2013; 91: 493–502.
Acknowledgements
This project was made possible by a grant from the National Organization for Rare Disorders (NORD). Additional support for this work was provided by the National Institutes of Health (1K08CA160662-01A1). We would like to thank Immunomedics, Inc. for generously providing the Wi2 anti-idiotype antibody to detect CAR expression. We would like to thank Dr John Morgan and Roger Williams Medical Center Core Facility for providing us with the necessary equipment to carry out flow cytometry and in vivo bioluminescence experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Katz, S., Point, G., Cunetta, M. et al. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther 23, 142–148 (2016). https://doi.org/10.1038/cgt.2016.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2016.14
This article is cited by
-
Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments
Journal of Translational Medicine (2023)
-
Immune profile of patients with peritoneal carcinomatosis selected for CRS-HIPEC therapy
Cancer Immunology, Immunotherapy (2023)
-
Systemic enhancement of antitumour immunity by peritumourally implanted immunomodulatory macroporous scaffolds
Nature Biomedical Engineering (2022)
-
Counteracting CAR T cell dysfunction
Oncogene (2021)
-
Monocytic and granulocytic myeloid-derived suppressor cell plasticity and differentiation are organ-specific
Oncogene (2021)