Abstract
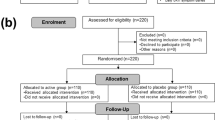
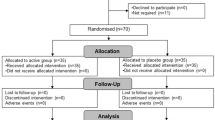
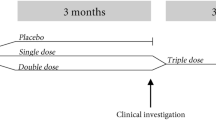
Use of probiotic-containing foods and probiotic supplements is increasing; however, few studies document safety and tolerability in conjunction with defined clinical end points. This paper reports the effects of 150 days of supplementation with either a single- (Bifidobacterium animalis subsp. lactis Bl-04) or a double-strain (Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis Bi-07) probiotic on routine haematology and clinical chemistry measures in healthy active adults. Pre- to post-intervention changes in laboratory measures were determined and compared between supplement and placebo groups. Overall there were few differences in routine haematology and clinical chemistry measures between supplement and placebo groups post-intervention. Exceptions included plasma calcium (P=0.03) and urea (P=0.015); however, observed changes were small and within assay-specific laboratory reference ranges. These data provide evidence supporting the use of these probiotic supplements over a period of 5 months in healthy active adults without obvious safety or tolerability issues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A . Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr 2013; 109 (Suppl 2): S35–S50.
Howarth GS, Wang H . Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 2013; 5: 58–81.
West NP, Pyne DB, Peake JM, Cripps AW . Probiotics, immunity and exercise: a review. Exerc Immunol Rev 2009; 15: 107–126.
Hoffmann DE, Fraser CM, Palumbo FB, Ravel J, Rothenberg K, Rowthorn V et al. Science and regulation. Probiotics: finding the right regulatory balance. Science 2013; 342: 314–315.
West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr 2013; 33: 581–587.
Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol 2011; 45: 518–525.
Acknowledgements
We thank the participants for their involvement and time, and staff at the Australian Institute of Sport, particularly Ms Anna Neumaier. This study was funded by Danisco Sweeteners OY, now part of DuPont.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Markus J Lehtinen and Sampo J Lahtinen are employees of Danisco Sweeteners Oy. Allan W Cripps is the recipient of research funding from Danisco Sweeteners Oy. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cox, A., West, N., Horn, P. et al. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur J Clin Nutr 68, 1255–1257 (2014). https://doi.org/10.1038/ejcn.2014.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.137
This article is cited by
-
A review of probiotic supplementation in healthy adults: helpful or hype?
European Journal of Clinical Nutrition (2019)
-
Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis
European Journal of Nutrition (2018)