Abstract
Beckwith–Wiedemann syndrome (BWS) is a model disorder for the study of imprinting, growth dysregulation, and tumorigenesis. Unique observations in this disorder point to an important embryonic developmental window relevant to the observations of increased monozygotic twinning and an increased rate of epigenetic errors after subfertility/assisted reproduction.
Similar content being viewed by others
In brief
-
Beckwith–Wiedemann syndrome (BWS) is a disorder of growth regulation exhibiting somatic overgrowth and a predisposition to embryonal tumors.
-
The incidence of BWS is estimated to be 1 out of 13 700.
-
Current tumor surveillance protocols include abdominal ultrasounds and alpha-fetoprotein (AFP) assays.
-
BWS is caused by various epigenetic and/or genetic alterations that dysregulate the imprinted genes on chromosome 11p15.5.
-
The BWS molecular subgroups are associated with different recurrence risks.
-
subfertility/assisted reproduction is associated with an increased frequency of BWS.
Introduction
Beckwith–Wiedemann syndrome (BWS) is a pediatric overgrowth disorder involving a predisposition to tumor development. The clinical presentation is highly variable; some cases lack the hallmark features of exomphalos, macroglossia, and gigantism as originally described by Beckwith and Wiedemann.1, 2 BWS is a panethnic disorder with an estimated population incidence of 1 in 13 700. This figure is likely an underestimate as milder phenotypes may not be ascertained. The incidence is equal in males and females with the notable exception of monozygotic twins that show a dramatic excess of females. In addition to clinical heterogeneity, BWS also exhibits etiologic molecular heterogeneity. A variety of genetic and/or epigenetic alterations in growth regulatory genes on chromosome 11p15.5 are associated with specific phenotype–epigenotype/genotype correlations including different recurrence risks. BWS usually occurs sporadically (85%), but familial transmission occurs in ∼15% of cases.
Clinical overview
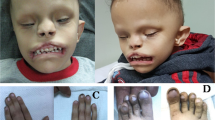
Individuals with BWS may grow at an increased rate during the latter half of pregnancy and in the first few years of life. Growth parameters typically show height and weight around the 97th percentile with head size closer to the 50th percentile. Adult heights are generally in the normal range.3, 4 Abnormal growth may also manifest as hemihyperplasia and/or macroglossia; the latter can lead to difficulties in feeding, speech and less frequently, sleep apnea. A recognizable facial gestalt is common and may include prominent eyes with infraorbital creases, facial nevus flammeus, midfacial hypoplasia, macroglossia, full lower face with a prominent mandible, anterior earlobe creases and posterior helical pits3 (Figure 1). The BWS facies often normalizes across childhood so that evaluation of adolescents or adults suspected to have BWS benefits from assessment of early childhood photographs. Development is usually normal unless there is chromosome 11p15.5 duplication or serious perinatal complications, including prematurity or uncontrolled hypoglycemia. Hypoglycemia is reported in 30–50% of babies with BWS,3, 5 likely caused by islet cell hyperplasia and hyperinsulinemia.
Individuals with BWS have an increased frequency of malformations and medical complications, including abdominal wall defects (omphalocele, umbilical hernia, and diastasis recti); visceromegaly involving any single or combination of organs: liver, spleen, pancreas, kidneys, and adrenals. Fetal adrenocortical cytomegaly is a pathognomonic finding for BWS. Unilateral or bilateral renal anomalies may include primary malformations, renal medullary dysplasia, nephrocalcinosis, and nephrolithiasis.6, 7, 8 Cardiac malformations are found in ∼20% of children with BWS; approximately half manifest cardiomegaly that resolves spontaneously.3, 9 Cardiomyopathy is rare.
Of major concern in children with BWS is the predisposition to embryonal malignancies. Most of the tumors associated with BWS occur in the first 8–10 years of life with very few being reported beyond this age;10, 11 most common are Wilms tumor and hepatoblastoma. Other embryonal tumors include rhabdomyosarcoma, adrenocortical carcinoma, and neuroblastoma.3, 10, 11, 12, 13, 14 A variety of other malignant and benign tumors have been reported.12, 13, 14 The overall risk for tumor development in children with BWS has been estimated at 7.5% with a range of risk estimates between 4 and 21%.3, 10, 11, 12, 13, 15, 16, 17 Clinical findings associated with higher risks of tumor development include hemihyperplasia, nephromegaly, and nephrogenic rests.10, 18 Although different molecular subgroups have been shown to be associated with different tumor rates and tumor profiles,19, 20, 21, 22, 23, 24 further clinical studies are needed to validate these data before implementing stratified surveillance protocols.
The BWS phenotype can, as noted above, vary significantly; for example, the diagnosis may be considered in a child presenting only with hemihyperplasia and nevus flammeus or possible ear creases, whereas the severe end of the spectrum may involve intrauterine, neonatal, or pediatric death. Death may be due to complications arising from hypoglycemia, prematurity, cardiomyopathy, macroglossia, or tumors. Potential causes for the variability in expression are discussed below.
Differential diagnosis
The presentation of a newborn with large growth parameters, macroglossia, and/or hypoglycemia, should prompt a comprehensive clinical examination followed by relevant investigations, for example, for maternal diabetes mellitus. Several genetic syndromes have features in common with BWS but can be distinguished by clinical genetics consultation, ancillary tests (eg, brain imaging, molecular and/or biochemical testing) and ongoing follow-up. The differential diagnosis includes Simpson–Golabi–Behmel syndrome, Costello syndrome, Perlman syndrome, Sotos syndrome, and mucopolysaccaridosis type VI (Maroteaux–Lamy syndrome), as well as mosaicism for trisomy 8. For cases involving asymmetry as an isolated finding, it is important to determine whether the asymmetry represents overgrowth (hemihyperplasia) or decreased growth (hemihypoplasia) as the latter is not known to be associated with an increased risk for tumor development. Molecular testing may provide clarification25.
Diagnostic approaches
Beckwith–Wiedemann syndrome is only one of several clinical conditions associated with epigenetic–genetic alterations on chromosome 11p15. This spectrum includes children with features of BWS who do not fulfill clinical diagnostic criteria, as well as children with isolated hemihyperplasia (IH)26, 27, 28, 29, 30 or isolated Wilms tumor.30
There are no absolute requisites for the clinical diagnosis of BWS. It is generally accepted that the presence of at least three major findings, or two major findings and one minor finding support a clinical diagnosis (Table 1). However, it is important to acknowledge the heterogeneous expression of this disorder and the role of positive molecular tests that may confirm the diagnosis even in cases with few cardinal clinical features. In this regard, epigenetic alterations of chromosome 11p15 in the lymphocytes of children with isolated Wilms tumor have recently been reported.30 Anticipatory medical management, especially with respect to tumor surveillance should be considered even for ‘milder’ presentations, for example, prominent tongue and umbilical hernia. As well, a proportion of cases of IH, a clinical diagnosis distinct from BWS, show molecular etiologies overlapping with BWS and parallel risks for embryonal tumor development.27 Molecular testing may be helpful in confirming the diagnosis of BWS but cannot at this time, rule it out. This is because a negative test result may only reflect a failure to detect the underlying molecular alteration in the tissue sampled. It is well-known that somatic mosaicism accounts for some of the BWS-associated clinical variability (such as asymmetry). It is important not to base medical management on data such as methylation indices or percentage of uniparental disomy (UPD) in the tissue tested (eg, blood), as this may not reflect the frequency of abnormal cells in the organs at risk for tumor development (eg, kidney).
In the case of a clinical diagnosis of BWS, molecular determination of altered methylation, a microdeletion at imprinting center 1 (IC1) and/or IC2, or a mutation in CDKN1C would confirm the diagnosis. However, if an epigenetic alteration is detected in a child with IH, the diagnostic assignment should be based on the clinical presentation; that is, IH should not be reclassified as BWS on the basis of a positive molecular diagnosis. Of note, epigenetic alterations in 11p15 including somatic mosaicism for paternal UPD for 11p15 and methylation alterations at IC1 or IC2 have been reported in IH with or without embryonal tumors.26, 27, 28, 29
Molecular basis of BWS
Most mammalian autosomal genes are expressed from both the maternally and paternally inherited copies of a chromosome pair. However, some genes undergo genomic imprinting and are expressed monoallelically in a parent-of-origin-specific manner. Genomic imprinting is regulated by epigenetic mechanisms (extrinsic to changes in primary nucleotide sequence) that include DNA methylation, histone modification, and noncoding RNAs. ICs are regions of DNA that regulate the expression of imprinted genes in cis over large distances and show differential methylation of the parental alleles. Therefore, they are also termed differentially methylated regions (DMRs).
The regulation of imprinted genes on chromosome 11p15.5 is shown in Figure 2. Deregulation of imprinted genes in the 11p15.5 imprinted region results in the BWS phenotype through a number of different mechanisms leading to either primary epigenetic alterations or genetic alterations that change the relative contributions of parental alleles.24, 31, 32 These include parent-of-origin-specific duplications, translocations/inversions, microdeletions, DNA methylation changes at IC1 or IC2, UPD, and mutations at CDKN1C. UPD refers to the presence of two chromosomal regions from one parent and none from the other.
(a) Schematic representation of the chromosome 11p15.5 imprinted region that is functionally divided into two domains. In the distal domain 1 are two imprinted genes, H19 and insulin-like growth factor 2 (1GF2). IGF2 is a paternally expressed fetal growth factor and H19 is a noncoding RNA. The H19-associated imprinting center (IC1) is usually methylated on the paternal chromosome and unmethylated on the maternal chromosome. Normally, the H19 gene is expressed from the maternal allele and IGF2 from the paternal allele. Domain 2 contains several imprinted genes, including KCNQ1, KCNQ1OT1, and CDKN1C. A differentially methylated region (IC2) contains the promoter for KCNQ1OT1, a paternally expressed noncoding transcript that regulates in cis the expression of the maternally expressed imprinted genes in domain 2. Two examples of imprinting alterations leading to Beckwith–Wiedemann syndrome (BWS) are shown in (b1) and (b2). (b1) IC1 gain of methylation in BWS is found in ∼5% of patients and leads to biallelic expression of IGF2. (b2) Loss of methylation at the KvDMR differentially methylated region (IC2) is found in 50% of BWS patients. This epigenetic alteration leads to reduced expression of CDKN1C. Red corresponds to preferential maternal allelic expression, blue corresponds to preferential paternal allelic expression. Filled rectangles indicate expressed genes and empty rectangles indicate non-expressed gene. Lollipops correspond to methylated sites.
Sporadic loss of methylation at IC2 occurs in 50% of patients.33 Gain-of-methylation defects occur at IC1 (5%); some of these methylation alterations have been associated with genomic alterations.34, 35, 36 Methylation changes that occur in conjunction with genomic alterations are important because of their heritability. Epigenetic alterations that involve both IC1 and IC2 generally indicate paternal UPD (20% of cases) for a chromosomal segment including 11p15.5.24, 33 Segmental UPD arises from a post-zygotic somatic recombination event and therefore has a mosaic distribution.
Methylation-sensitive multiplex ligation probe analysis (MS-MLPA) is currently the most robust method for detecting the majority of epigenetic and genetic etiologies associated with BWS. It detects microdeletions/microduplications, alterations in gene dosage, and DNA methylation including UPD.37 Confirmation of UPD11 may be undertaken by analysis of short tandem repeats as the somatic mosaicism associated with this etiology may lead to weak signals on MS-MLPA. Moreover, failure to detect UPD11 in one tissue (usually leukocytes) is not conclusive. One should consider obtaining another tissue (such as skin), especially in the event of surgery. Karyotype analysis will detect the rare de novo and maternally transmitted translocations/inversions (1%) and paternally derived duplications (1%) of chromosome 11p15.5 associated with BWS. Translocation/inversions almost always disrupt the gene, KCNQ1,38 and are not usually detectable by MLPA because most do not show DNA copy number changes or DNA methylation changes. Finally, DNA sequencing is required to detect genomic alterations in CDKN1C associated with BWS. The CDKN1C (p57kip2) mutations are seen both sporadically (5%) and in autosomal dominant pedigrees modified by preferential parent of origin-specific transmission (40%).39 A rational clinical approach to test these varied defects on chromosome 11p15.5 is presented in Figure 3.
Current studies of BWS patient cohorts identify a chromosome 11p15 molecular alteration in only 75–80% of individuals. Therefore, other genomic loci are likely involved in the etiology of BWS.40 Recent molecular studies have shown two genes, NALP2 on chromosome 1941 and ZFP57 on chromosome 642 that can modulate imprinting at IC2.
Clinical findings relevant to molecular etiology
Tumor development
Individuals with UPD of 11p15.5 or gain of methylation at the H19 IC carry the highest risk to develop Wilms tumor or hepatoblastoma.19, 20, 21, 22, 23, 24, 30 Those with loss of maternal methylation at IC2 carry a lower tumor risk; as well, the tumors in this molecular subgroup do not include Wilms tumor. Lastly, individuals with mutations in CDKN1C seem to have the lowest risk with only a small number of cases reported. In cases with CDKN1C mutation, only neuroblastoma has been reported to date.
Hemihyperplasia in cases of BWS
Alterations have included mosaicism for UPD 11p15.5 with hemihyperplasia and or molecular alterations at IC2 or IC1.32, 43
Positive family history
This is associated with mutations in CDKN1C or microdeletions of IC1 and very rarely IC2.32, 33, 35, 36, 37, 40, 44, 45
Omphalocele
This is associated with an IC2 defect or CDKN1C mutation.32
Developmental delay
This is associated with cytogenetically detectable duplications involving the paternal copy of chromosome 11p15.5.46, 47
A severe BWS phenotype
This seems to be associated, at least in certain cases, with very high levels of paternal chromosome 11p15.48
An increased frequency of female monozygotic twins discordant for BWS has been reported.49 These females usually show loss of methylation at IC2. In contrast, the less frequently observed male monozygotic twins show a broad spectrum of BWS-associated molecular alterations.48
Subfertility/assisted reproductive technologies (ARTs)
seem to be associated with an increased risk of BWS cases with loss of methylation at IC2 (Figure 4).43, 50, 51, 52 No specific aspect of subfertility or its treatment has been specifically associated with the increased risk of epigenetic defects associated with BWS after ART.
Enrichment of epigenetic defects in Beckwith–Wiedemann (BWS). In the general population, loss of methylation (LOM) at KvDMR on chromosome 11p15.5 contributes to 50% of BWS cases, whereas in the BWS/ART population, LOM at KvDMR is found in ∼95% of cases. This represents a 1.9-fold enrichment of this epigenetic defect in the BWS/ART population.
Management treatment and care
The management of BWS patients typically involves standard supportive medical and surgical strategies (eg, surgical repair of omphalocele). In addition, anticipatory medical management for certain findings should be invoked if the diagnosis of BWS is established or even suspected. If there are prenatal findings suggestive of or diagnostic for BWS,53 screening for hypoglycemia should be undertaken in the first few days of life. As well, parents should be advised of the typical clinical manifestations of hypoglycemia in the event that it manifests after discharge from hospital.
Tumor surveillance should be initiated if BWS is diagnosed or suspected. Current tumor surveillance protocols may vary among different centers in terms of frequency and duration.11, 54, 55, 56 Abdominal ultrasounds are used to assess kidneys, liver, pancreas, and adrenal glands; in many centers this is undertaken quarterly to the age of 8 years.56 In the event that ultrasound imaging shows a suspicious finding, for example, a lesion possibly representing a hemangioma, computed tomography or magnetic resonance imaging would be indicated for better resolution. As well, baseline magnetic resonance imaging has been suggested54, 57 to provide information for subsequent interpretation of imaging. Reports of large organs may not merit consultation with pediatric specialists as these are not infrequently found in BWS; however, other findings including renal cysts or nephrogenic rests should be carefully investigated by the appropriate specialists. Any concern on physical examination or imaging should be investigated immediately along with the consideration of referral, for example, pediatric oncology.
As part of tumor surveillance, alpha-fetoprotein (AFP) can be measured periodically to the age of 4 years for early detection of hepatoblastoma. Guidelines for the intervals between AFP assays vary between 2 and 3 months.11, 31, 58 It should be noted that AFP levels tend to be increased in infants with BWS.59 In the event of an increased AFP level, a repeat study should be undertaken in 4 weeks along with baseline liver function studies and correlation with abdominal imaging. If the AFP level is increasing, focused investigation for an underlying tumor should be undertaken in consultation with a pediatric oncologist. If the AFP is falling and imaging and liver function studies are normal, the AFP should be checked every 4–6 weeks until it falls to within the normal range. As the risk for neuroblastoma is small, specific surveillance including quarterly urine catecholamines and annual chest X-ray is not currently recommended but rather is left to the discretion of the responsible physician.11 Tumor surveillance is recommended for the apparently unaffected monozygotic co-twin (determined by molecular testing) of a child with BWS even in the absence of any clinical stigmata or a negative molecular test result in the co-twin. This is primarily indicated because of possible somatic mosaicism in the normal co-twin or seeding of BWS positive cells in the normal co-twin secondary to common vascular anastomoses in utero.
Other medical issues, including hypoglycemia, abdominal wall defects, and renal abnormalities, are managed as in children without BWS involving specialists as appropriate. The onset of nephrocalcinosis or nephrolithiasis because of medullary sponge kidney may be delayed into adolescence and ongoing annual renal ultrasound surveillance into mid-adolescence is reasonable. Any concern about a cardiac abnormality should be investigated promptly and especially before any planned surgery. Cardiomyopathy is rare and was recently reported in conjunction with high levels of UPD in two cases of BWS.48
With respect to growth, there is no treatment indicated for macrosomia as final growth parameters are usually within normal limits; however, growth parameters should be followed regularly. If hemihyperplasia is present and involves the legs and/or trunk, screening for scoliosis should be included in routine physical examinations. Ongoing follow-up by an orthopedic surgeon should be organized for leg-length discrepancies greater than 1–2 cm and epiphysiodesis of the longer leg may be considered at the appropriate time. In contrast to some overgrowth disorders involving a region of the body or a limb, the degree of hemihyperplasia typically does not dramatically change during childhood and parents often find this information reassuring. Macroglossia, if mild-to-moderate in presentation, may be accommodated within the oral cavity as the facial bone structures grow in infancy and early childhood. This is best assessed by a craniofacial team, including plastic surgeons, speech pathologists, and orthodontists. Macroglossia may pose difficulties for feeding and/or speech articulation and referral to feeding specialists and speech pathologists may be helpful. Surgical reduction and/or mandibular surgery may be considered if orthodontic and/or cosmetic issues are of concern. However, surgery typically does not impact the thickness of the tongue and residual cosmetic and/or speech issues may remain.60 Consultation with respirologists and consideration of sleep studies may be indicated if there is concern regarding sleep apnea.
Genetic counseling
Genetic counseling regarding etiology and recurrence risks for BWS is most accurate if data from a complete diagnostic evaluation are available, including current molecular testing (that is, clinical findings, karyotype, MS-MLPA, and CDKN1C mutation analysis if indicated). Information regarding recurrence risks for some of the molecular subtypes remains theoretical rather than definitive as there are no confirmatory empiric data. This is especially relevant when providing genetic counseling for affected individuals with theoretically low recurrence risks, for example, with UPD, methylation alterations at IC2. Molecular testing is typically not indicated for parents or other family members when UPD is found, as these cases arise through post-zygotic somatic recombination. Parental studies are recommended if genomic alterations are found, that is, karyotype abnormalities, CDKN1C mutations or microduplications or microdeletions of the 11p15.5 region.
Molecular alterations that may be associated with high recurrence risks include: maternal transmission of 11p15.5 translocations3 or CDKN1C mutations,40 11p15.5 duplications of paternal origin,46 and 11p15.5 microdeletions.36 This is also true for positive family histories in the absence of an abnormal genetic test result. In such families, the recurrence risk is determined by the sex and potential carrier status of the transmitting parent. The recurrence risk for BWS in these cases may be as high as 50%. For CDKN1C mutations and for translocations or inversions involving chromosome 11p15.5, the recurrence risk would be 50% if the transmitting parent was the mother.40 Molecular testing or chromosome analysis is indicated for both the parents and potentially other family members if either of these abnormalities are found in the proband. If the CDKN1C mutation is found in the father, a specific recurrence risk figure cannot be quoted; this risk is felt to be low but at least one case of paternal transmission has been reported in the literature.44 As well, the recurrence risk for paternally derived duplications has not been specifically defined but is likely significant if the father carries a translocation. Familial transmissions involving microdeletions of IC1 and one case of IC2 have been reported and parental testing is indicated35, 36, 37 (Figure 1). Gonadal mosaicism should be considered in the provision of recurrence risks when parents are not found to carry a transmissable microdeletion or mutation associated with BWS.
Genetic testing strategy
Prenatal diagnosis
Some families may choose to pursue prenatal testing especially if they have had a child with severe manifestations of BWS. If a cytogenetic or genomic abnormality (eg, microdeletion, CDKN1C mutation) has been identified, prenatal testing may be indicated by CVS or amniocentesis; if such abnormalities are de novo, gonadal mosaicism remains a possibility. Epigenetic analysis of amniocytes can be undertaken by MS-MLPA. Families, for whom UPD or methylation alterations have been detected in the absence of a transmissible genomic alteration, may also wish to consider prenatal diagnostic testing even though the recurrence risk would be very low. Of note, a recent follow-up study of apparently isolated fetal omphalocele reported BWS in 20% of cases based on clinical or molecular findings.53
In the absence of a known molecular defect, measurement of maternal serum AFP can be offered. As well, in all at risk pregnancies, prenatal screening can be offered including: nuchal translucency measurement between 10 and 14 weeks, detailed ultrasound at 18–20 weeks and again at 25–32-weeks gestation. Ultrasound should be directed at assessment of growth parameters that may become advanced for gestational age (usually after 24 weeks), abdominal wall defects, organomegaly, renal abnormalities, cleft palate, cardiac anomalies, and macroglossia.
Conclusion
Over the next few years, we expect that much progress will be made in research on BWS. In order to translate these data rapidly to the clinical arena, it will be important to periodically review the status of both clinical and molecular aspects of BWS. Clinical diagnosis, molecular testing, genetic counseling, and management will likely all be impacted. The available clinical data may improve as we develop better protocols for complete ascertainment of BWS cases and as the total number of cases studied continues to rise. Further, MS-MLPA and other testing modalities will more accurately reflect the total number of BWS cases with identified molecular lesions, although there will likely be new molecular etiologies identified for both familial and sporadic cases.
References
Beckwith JB : Abstract, Western Society for Pediatric Research 1963; Extreme cytomegaly of the adrenal fetal cortex, omphalocele, hyperplasia of kidneys and pancreas, and Leydig-cell hyperplasia: Another syndrome? Los Angeles, November 11.
Wiedemann HR : Complexe malformatif familial avec hernie ombilicale et macroglossia, un ‘syndrome nouveau. J Genet Hum 1964; 13: 223–232.
Pettenati MJ, Haines JL, Higgins RR, Wappner RS, Palmer CG, Weaver DD : Wiedemann–Beckwith syndrome: presentation of clinical and cytogenetic data on 22 new cases and review of the literature. Hum Genet 1986; 74: 143–154.
Weng EY, Moeschler Jr JB, Graham JM : Longitudinal observations on 15 children with Wiedemann–Beckwith syndrome. Am J Med Genet 1995a; 56: 366–373.
Engstrom W, Lindham S, Schofield P : Wiedemann–Beckwith syndrome. Eur J Pediatri 1988; 147: 450–457.
Choyke PL, Siegel MJ, Oz O, Sotelo-Avila C, De Baum MR : Nonmalignant renal disease in pediatric patients with Beckwith–Wiedemann syndrome. AJR Am J Roentgenol 1998; 171: 733–737.
Borer JG, Kaefer M, Barnwolt CE et al: Renal findings on radiological follow-up of patients with Beckwith–Wiedemann syndrome. J Urol 1999; 161: 235–239.
Goldman M, Smith A, Shuman C et al: Renal abnormalities in Beckwith–Wiedemann syndrome are associated with 11p15.5 uniparental disomy. J Am Soc Nephrol 2002; 13: 2077–2084.
Elliott M, Maher ER : Beckwith–Wiedemann syndrome. J Med Genet 1994; 31: 560–564.
DeBaun MR, Siegel MJ, Choyke PL : Nephromegaly in infancy and early childhood: a risk factor for Wilms tumor in Beckwith–Wiedemann syndrome. J Pediatr 1998; 132: 401–404.
Tan TY, Amor DJ : Tumour surveillance in Beckwith–Wiedemann syndrome and hemihyperplasia: a critical review of the evidence and suggested guidelines for local practice. J Paed Child Health 2006; 42: 486–490.
Sotelo-Avila C, Gonzalex-Crussi F, Fowler JW : Complete and incomplete forms of Beckwith–Wiedemann syndrome: their oncogenic potential. J Pediatr 1980; 96: 47–50.
Wiedemann HR : Tumors: an hemihypertrophy associated with Wiedemann–Beckwith syndrome. Eur J Pediatr 1983; 141: 129.
Lapunzina P : Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am J Med Genet 2005; 137: 53–71.
Elliott M, Bayly R, Cole T, Temple IK, Maher ER : Clinical features and natural history of Beckwith–Wiedemann syndrome: presentation of 74 new cases. Clin Genet 1994; 46: 168–174.
Weng EY, Mortier GR, Graham Jr JM : Beckwith–Wiedemann syndrome. An update and review for the primary pediatrician. Clin Pediatr (Phila) 1995; 34: 317–326.
Schneid H, Vazquez MP, Vacher C, Gourmelen M, Cabrol S, Le Boucs Y : The Beckwith–Wiedemann syndrome phenotype and the risk of cancer. Med Paediatr Oncol 1997; 28: 411–415.
Coppes MJ, Arnold M, Beckwith JB et al: Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer 1999; 85: 1616–1625.
Weksberg R, Nishikawa J, Caluseriu O et al: Tumor development in the Beckwith–Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alternations including imprinting defects of KCNQ1OT1. Hum Mol Genet 2001; 10: 2989–3000.
Bliek J, Maas SM, Ruijter JM et al: Increased tumour risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 methylation: occurrence of KCNQ1OT1 hypomethylation in familial cases of BWS. Hum Mol Genet 2001; 10: 467–476.
Bliek J, Gicquel C, Maas S, Gaston V, Le Bouc Y, Mannens M : Epigenotyping as a toll for the predicition of tumor risk and tumor type in patients with Beckwith–Wiedemann syndrome (BWS). J Pediatr 2004; 145: 796–799.
DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP : Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith–Wiedemann syndrome with cancer and birth defects. Am J Hum Genet 2002; 70: 604–611.
Rump P, Zeegers MP, van Essen AJ : Tumor risk in Beckwith–Wiedemann syndrome: a review and meta-analysis. Am J Med Genet 2005; 136: 95–104.
Cooper WN, Luharia A, Evans GA et al: Molecular subtypes and phenotypic expression of Beckwith–Wiedemann syndrome. Eur J Hum Genet 2005; 13: 1025–1032.
Eggermann T, Schönherr N, Meyer E et al: Epigenetic mutations in 11p15 in Silver–Russell syndrome are restricted to the telomeric imprinting domain. J Med Genet 2006; 43: 615–616.
Bliek J, Maas S, Alders M, Merks JH, Mannens M : Epigenotype, phenotype, and tumors in patients with isolated hemihyperplasia. J Pediatr 2008; 153: 95–100.
Shuman C, Smith AC, Steele L et al: Constitutional UPD for chromosome 11p15 in individuals with isolated hemihyperplasia is associated with high tumor risk and occurs following assisted reproductive technologies. Am J of Med Genet 2006; 140: 1497–1503.
Martin RA, Grange DK, Zehnbauer B, Debaun MR : LIT1 and H19 methylation defects in isolated hemihyperplasia. Am J Med Genet 2005; 134A: 129–131.
Grundy P, Telzerow P, Paterson MC et al: Chromosome 11 punparental isodisomy predisposing to embryonal neoplasms. Lancet 1991; 338: 1079–1080.
Scott RH, Douglas J, Baskcomb L et al, Factors Associated with Childhood Tumours (FACT) Collaboration, Cook JA, Pujol P, Maher ER, Birch JM, Stiller CA, Pritchard-Jones K, Rahman N: Constitutional 11p15 abnormalities, including heritable imprinting center mutations, cause nonsyndromic Wilms tumor. Nat Genet 2008; 40: 1329–1334.
Weksberg R, Shuman C, Smith AC : Beckwith–Wiedemann Syndrome. Am J of Med Genet 2005; 137: 12–23.
Enklaar T, Zabel BU, Prawitt D : Beckwith–Wiedemann syndrome: multiple molecular mechanisms. Expert Rev Mol Med 2006; 8: 1–19.
Weksberg R, Shuman C : Beckwith–Wiedemann syndrome and hemihypertrophy; In: Cassidy SB, Allanson JE (eds): Management of Genetic Syndromes, 2nd ed. New York: John Wiley & Sons, Inc, 2000, pp 101–116.
Niemitz EL, DeBaun MR, Fallon J et al: Microdeletion of LIT1 in familial Beckwith–Wiedemann syndrome. Am J Hum Genet 2004; 75: 844–849.
Sparago A, Cerrato F, Vernucci M, Ferrero GB, Silengo MC, Riccio A : Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith–Wiedemann syndrome. Nat Genet 2004; 36: 958–960.
Prawitt D, Enklaar T, Gärtner-Rupprecht B et al: Microdeletion of target sites for insulator protein CTCF in a chromosome 11p15 imprinting center in Beckwith–Wiedemann syndrome and Wilms tumor. Proc Natl Acad Sci USA 2005; 102: 4085–4090.
Scott RH, Douglas J, Baskcomb L et al: Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) robustly detects and distinguished 11p15 abnormalities associated with overgrowth and growth retardation. J Med Genet 2008; 45: 106–113.
Smilinich NJ, Day CD, Fitzpatrick GV et al: A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith–Wiedemann syndrome. Proc Natl Acad Sci USA 1999; 96: 8064–8069.
Li M, Squire J, Shuman C et al: Imprinting status of 11p15 genes in Beckwith–Wiedemann syndrome patients with CDKN1C mutations. Genomics 2001; 74: 370–376.
Bliek J, Verde G, Callaway J et al: Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith–Wiedemann syndrome. Eur J Hum Genet 2009; 17: 611–619.
Meyer E, Lim D, Pasha S et al: Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith–Wiedemann syndrome). PLOS Genet 2009; 5: 1–5.
Mackay DJ, Callaway JL, Marks SM et al: Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet 2008; 40: 949–951.
DeBaun MR, Niemitz EL, Feinberg AP : Association of in vitro fertilization with Beckwith–Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003; 72: 156–160.
Lee MP, DeBaun M, Randhawa G, Reichard BA, Elledge SJ, Feinberg AP : Low frequency of p57KIP2 mutation in Beckwith–Wiedemann syndrome. Am J Hum Genet 1997; 61: 304–309.
Hatada I, Nabetani A, Morisaki H et al: New p57KIP2 mutations in Beckwith–Wiedemann syndrome. Hum Genet 1997; 100: 681–683.
Waziri M, Patil SR, Hanson JW, Bartley JA : Abnormality of chromosome 11 in patients with features of Beckwith–Wiedemann syndrome. J Pediatr 1983; 102: 873–876.
Slavotinek A, Gaunt L, Donnai D : Paternally inherited duplications of 11p15.5 and Beckwith–Wiedemann syndrome. J Med Genet 1997; 34: 819–826.
Smith AC, Rubin T, Shuman C et al: New chromosome 11p15 epigenotypes identified in male monozygotic twins with Beckwith–Wiedemann syndrome. Cytogenet Genome Res 2006; 113: 313–317.
Weksberg R, Shuman C, Caluseriu O et al: Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith–Wiedemann syndrome. Hum Mol Genet 2002; 15: 1317–1325.
Gicquel C, Caston V, Mandelbaum J : In vitro fertilization may increase the risk of Beckwith–Wiedemann syndrome related to the abnormal imprinting of the KCNQ1OT gene. Am J Hum Genet 2003; 72: 1338–1341.
Maher ER, Afnan M, Barratt C : Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod 2003; 17: 2508–2511.
Halliday J, Oke K, Breheny S, Algar E, J Amor D : Beckwith–Wiedemann syndrome and IVF: a case–control study. Am J Hum Genet 2004; 75: 526–528.
Wilkins-Haug L, Porter A, Hawley P, Benson CB : Isolated fetal omphalocele, Beckwith–Wiedemann syndrome, and assisted reproductive technologies. Birth Defects Res A Clin Mol Teratol 2009; 85: 58–62.
Beckwith JB : Nephrogenic rests and the pathogenesis of Wilms tumor: developmental and clinical considerations. Am J Med Genet 1998b; 79: 268–273.
Rahman N, Craft AW, Kenney I et al: Surveillance for Wilms tumour in at-risk individuals – pragmatic recommendations for best practice. The Wilms Tumour Surveillance Working Group 2005; 19: Available from: http://www.bshg.org.uk/documents/other_docs/Wilms%20Tumour%20surveillance%20recommendations.pdf.
Weksberg R, Shuman C : Beckwith–Wiedemann syndrome and hemihypertrophy; In: Cassidy SB, Allanson JE (eds):Management of Genetic Syndromes, 3rd edn. New York: John Wiley & Sons, Inc, 2009, In Press.
Clericuzio CL, D'Angio GJ, Duncan M, Green DM, Knudson Jr AG : Summary and recommendations of the workshop held at the First International conference on molecular and clinical genetics of childhood renal tumors. Albuquerque, New Mexico. Med Pediatr Oncol 1992; 14–16: 233–236.
Clericuzio CL, Chen E, McNeil DE et al: Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith–Wiedemann syndrome or isolated hemihyperplasia. J Pediatr 2003; 143: 270–272.
Everman DB, Shuman C, Dzolganovski B, O'Riordan MA, Weksberg R, Robin NH : Serum alpha-fetoprotein levels in Beckwith–Wiedemann syndrome. J Pediatr 2000; 137: 123–127.
Tomlinson JK, Morse SA, Bernard SP, Greensmith AL, Meara JG : Long-term outcomes of surgical tongue reduction in Beckwith–Wiedemann syndrome. Plast Reconstr Surg 2007; 119: 992–1002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weksberg, R., Shuman, C. & Beckwith, J. Beckwith–Wiedemann syndrome. Eur J Hum Genet 18, 8–14 (2010). https://doi.org/10.1038/ejhg.2009.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2009.106
Keywords
This article is cited by
-
Hallmark discoveries in the biology of Wilms tumour
Nature Reviews Urology (2024)
-
Capturing sex-specific and hypofertility-linked effects of assisted reproductive technologies on the cord blood DNA methylome
Clinical Epigenetics (2023)
-
Clinical significance of nonerythrocytic spectrin Beta 1 (SPTBN1) in human kidney renal clear cell carcinoma and uveal melanoma: a study based on Pan-Cancer Analysis
BMC Cancer (2023)
-
EpiTyping: analysis of epigenetic aberrations in parental imprinting and X-chromosome inactivation using RNA-seq
Nature Protocols (2023)
-
Spectrum of congenital anomalies of the kidney and urinary tract (CAKUT) including renal parenchymal malformations during fetal life and the implementation of prenatal exome sequencing (WES)
Archives of Gynecology and Obstetrics (2023)