Abstract
Aims
Foreign materials used in ocular surface surgery may lead to local complications such as discomfort, scarring, or infection. Plasma-derived products such as fibrin glue may produce possible hypersensitivity reactions whereas the risk of viral transmission remains. We describe a simple method of achieving conjunctival autograft adherence during pterygium surgery avoiding potential complications associated with the use of fibrin glue or sutures.
Methods
After pterygium excision and fashioning of the autologous conjunctival graft, the recipient bed is encouraged to achieve natural haemostasis and relative dessication before graft placement. Excessive haemorrhage in the graft bed is tamponaded. Graft adherence and positioning is examined 20 min after surgery.
Results
A total of 15 eyes of 12 patients (mean (SD) age 73.7 (11.2) years), 8 females underwent SGF autologous conjunctival graft post-pterygium excision. Mean graft area was 24(1.5) mm2. Mean follow-up time was 9.2 (2.2) months. Cosmesis was excellent in all cases and visual acuity improved in one patient. There were no intra- or post-operative complications requiring further treatment.
Conclusion
This simple technique for pterygium surgery may prevent potential adverse reactions encountered with the use of foreign materials and in this small series provided safe and comparable results to current methods.
Similar content being viewed by others
Introduction
In 1985, Kenyon et al1 proposed that a conjunctival autograft of the bare sclera could be used in treatment of recurrent and advanced pterygium. Recent reports favour the use of fibrin glue2, 3, 4, 5 above sutures with improved comfort, decreased surgical time, reduced complication and recurrence rates have been reported. Suture-related complications include infection, granuloma formation, and chronic inflammation,6, 7 whereas plasma-derived fibrin glue has the potential risk of prion disease transmission and anaphylaxis in susceptible individuals. Sutureless ‘laissez-faire’ grafting has been used successfully in gingival grafts8 and represents a similar mucosal membrane tissue environment to the conjunctiva of the eye.
Materials and methods
Surgical technique
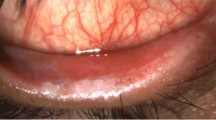
The body of the pterygium is dissected 4 mm from the limbus, down to bare sclera, and reflected over the cornea. The pterygium head and cap is avulsed using artery forceps followed by careful beaver blade (Hockey stick; Grieshaber, Fribourg, Switzerland) excision of corneal remnants. Only the thickened portions of the conjunctiva and the immediate adjacent and subjacent Tenon's capsule showing tortuous vasculature are excised. Care is taken to avoid conjunctival plica excision and extensive dissection of tenons is avoided. Where possible, haemostasis is allowed to occur spontaneously without the use of cautery. If no blood is available to provide autologous fibrin, small perforating veins and capillaries are purposely fractured (Figure 1b) (though seldom required) to encourage a thin layer of fresh blood to cover the bare sclera. The size of the defect (mm2) is measured with Castoviejo callipers (Bausch & Lomb Storz; Storz Instruments, St Louis, MO, USA).
Careful dissection between donor graft conjunctiva and Tenon's layer is used while fashioning the 1 mm oversized conjunctivo-limbal graft from the superior bulbar conjunctiva. The limbal edge of the graft is carefully positioned at the host limbal tissue edge as previously described.7 No attempt is made to directly close the full extent of the excision wound, allowing natural graft positioning without tension.
The scleral bed is viewed through the transparent conjunctiva and to ensure residual bleeding does not relift the graft, small central haemorrhages are tamponaded with direct compression using non-toothed forceps (Figure 1d) until haemostasis is achieved, usually within 8–10 min. The stabilisation of the graft is tested with a Merocel spear centrally and on each free edge to ensure firm adherence to sclera. Postoperatively (Figure 2), steroid drops were initially given four times a day and tapered over 6 weeks while antibiotic drops were administered four times a day for 1–2 weeks.
Cross sectional study
All records of patients undergoing pterygium surgery with conjunctival autografting at the Mater Hospital in Belfast between January 2008 and June 2009 were reviewed. All patients had primary nasal pterygia requiring surgical excision and signed informed consent. All patients received a suture and glue-free (SGF) autologous graft that was the preferred choice of surgical technique by one of the authors (JM) and has been previously described.9 Primary outcome measures included graft dislocation and pterygium recurrence. Secondary outcome measures included size of graft used, patient comfort on visual analogue scale, and visual acuity. Patients who were still registered with the hospital and living within reasonable commuting distance were contacted by telephone and asked to attend a voluntary follow-up examination in late August 2009. Those who were able to attend were then examined by one investigator (DDW) using a Haag–Streit slit lamp and after signed consent for photography, anterior segment camera photographs were taken to assess for pterygium recurrence, complications, and cosmetic result. Recurrence was defined as the presence of fibrovascular tissue regrowth extending beyond the surgical limbus onto clear cornea as agreed by Sebban and Hirst.10
All methods adhered to the tenets of the Declaration of Helsinki Principles for research in human subjects. Pre-operative and post-operative visual acuities together with complications were recorded from the patients records. Cosmesis at the final visit was judged by one investigator (DDW) according to the grading system reported by Prabhasawat et al.11 Post-operative pain intensity was assessed with a visual analogue score from 1 to 10 on the first post-operative day. The average post-operative pain within the first week was recorded retrospectively at the 1-week appointment. Subjective comments related to cosmesis were asked of each patient using a visual analogue score at the time of review.
Results
A total of 15 eyes of 12 patients (mean (SD) age 73.7 (11.2)) years underwent SGF autologous conjunctival graft after pterygium excision (Table 1). There were eight female and four male patients. All patients had primary nasal pterygium and signed informed consent. Mean follow-up time was 9.2 (2.2) months. Mean graft area was 24(1.5) mm.2 The mean surgical time was 14(1.4) min. There were no transplant dislocations or failures. There were no intra- or post-operative complications requiring further treatment. Visual acuities were not affected in the majority of patients. One patient with a large 5 mm pterygium encroaching 1 mm from the visual axis improved by 2 Snellen chart lines after surgery (6 of 24 to 6 of 12). Patients rated their cosmesis as excellent in all cases and photographic comparison of nasal to temporal conjunctiva at last review revealed no obvious cosmetic defects or recurrences. Post-operative pain on day 1 after surgery was consistently rated as less than or equal to 2 out of 10 on a visual analogue score. Pain did not increase after the first post-operative day.
Discussion
Current surgical methods to prevent pterygium recurrence include conjunctival autograft, limbal and limbal–conjunctival transplant, conjunctival flap and conjunctival rotation autograft surgery, amniotic membrane transplant, cultivated conjunctival transplant, lamellar keratoplasty, and the use of fibrin glue.12 All of these techniques involve the use of sutures or fibrin glue and are therefore vulnerable to associated complications.
The presence of sutures may lead to prolonged wound healing and fibrosis.4, 6 Subsequent complications such as pyogenic granuloma formation are easily treated; others such as symblepharon formation, forniceal contracture, ocular motility restriction, diplopia, scleral necrosis, and infection are much more difficult to manage and may be sight threatening.13, 14
Although generally considered safe, fibrin glues are currently manufactured from human plasma and therefore carry the theoretical risk of transmissible disease.12 Virus removal and inactivation procedures are included in the manufacturing process although may be of limited value against nonenveloped viruses such as hepatitis A virus and parvovirus B19.15 New devices, such as the CryoSeal FS System, that generate fibrin sealant from autologous blood may eliminate the current risks associated with pooled plasma. They are not currently in widespread use however and the time taken to procure the fibrin may be prohibitive in day case pterygium surgery.16 Fibrinogen compounds may also be susceptible to inactivation by iodine preparations such as those used for conjunctival disinfection before pterygium surgery.17 In this setting their superiority versus naturally occurring fibrin in the bare scleral wound site has not been directly compared. The apposition of the lids to the bulbar conjunctiva provides a natural biological dressing and confers a unique wound-healing environment. Apart from a physical barrier, the lids provide compression, a smooth frictionless surface, and a vascular bed with immune capability in close proximity to the injury site. Conjunctival healing rates of 3.16±0.17 mm2 per day have been shown in rabbit models18 and this in addition to the natural biological dressing afforded by lid closure appears uniquely adequate in allowing the use of free conjunctival grafts. Our study has several limitations. It was non-randomised and consisted of a small study population and a relatively short follow-up period of 9 months. However, one article comparing four commonly used techniques for pterygium surgery reported mean time for appearance of any complication including recurrence was 4 months.19 Most importantly however, the operating time, post-operative symptoms, recurrence, and complication rate of the above-described technique (SGF) in our series appears to be equivalent to conventional suture and glue techniques of a similar follow-up duration.3, 4, 6, 10 Specifically, the risk of graft retraction as described by Tan7 appears to be no greater without suturing or fibrin glue as long as meticulous dissection of the subepithelial graft tissue is respected. We postulate that as there is an even tension across the whole of the graft interface and no direct tension on the free graft edges, there is reduced stimulus for subconjunctival scar tissue to form. Although surgical time in our small series appears no greater than current published literature,20 the possibility of longer operation times compared to sutures or fibrin glue is possible. A prospective randomised controlled trial is required to investigate the long-term efficacy of this SGF grafting technique in reducing recurrences.
References
Kenyon KR, Wagoner MD, Hettinger ME . Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 1985; 92: 1461–1470.
Ayala M . Results of pterygium surgery using a biologic adhesive. Cornea 2008; 27: 663–667.
Kim HH, Mun HJ, Park YJ, Lee KW, Shin JP . Conjunctivolimbal autograft using a fibrin adhesive in pterygium surgery. Korean J Ophthalmol 2008; 22: 147–154.
Koranyi G, Seregard S, Kopp ED . Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol 2004; 88: 911–914.
Koranyi G, Seregard S, Kopp ED . The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmologica Scandinavica 2005; 83: 298–301.
Allan BD, Short P, Crawford GJ, Barrett GD, Constable IJ . Pterygium excision with conjunctival autografting: an effective and safe technique. Br J Ophthalmol 1993; 77: 698–701.
Tan D . Conjunctival grafting for ocular surface disease. Curr Opin Ophthalmol 1999; 10: 277–281.
Dorfman HS, Kennedy JE, Bird WC . Longitudinal evaluation of free autogenous gingival grafts. A four year report. J Periodontol 1982; 53: 349–352.
Sharma A, Moore J . Autologous fibrin glue for pterygium surgery with conjunctival autograft. Cont Lens Anterior Eye 2009; 32: 209.
Sebban A, Hirst LW . Pterygium recurrence rate at the Princess Alexandra Hospital. Aust NZJ Ophthalmol 1991; 19: 203–206.
Prabhasawat P, Barton K, Burkett G, Tseng SC . Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 1997; 104: 974–985.
Ang LP, Chua JL, Tan DT . Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol 2007; 18: 308–313.
Solomon A, Pires RT, Tseng SC . Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 2001; 108: 449–460.
Vrabec MP, Weisenthal RW, Elsing SH . Subconjunctival fibrosis after conjunctival autograft. Cornea 1993; 12: 181–183.
Groner A . Pathogen safety of plasma-derived products—Haemate P/Humate-P. Haemophilia 2008; 14 (Suppl 5): 54–71.
Buchta C, Dettke M, Funovics PT, Höcker P, Knöbl P, Macher M et al. Fibrin sealant produced by the CryoSeal FS System: product chemistry, material properties and possible preparation in the autologous preoperative setting. Vox Sang 2004; 86: 257–262.
Gilmore OJ, Reid C . Prevention of intraperitoneal adhesions: a comparison of noxythiolin and a new povidone–iodine/PVP solution. Br J Surg 1979; 66: 197–199.
Zhu X, Beuerman RW, Cheng ZY, Ang LPK, Tan DTH . Kinetic analysis of conjunctival epithelial wound healing in the rabbit model. Invest Ophthalmol Vis Sci 2005; 46: 4247.
Alpay A, Ugurbas SH, Erdogan B . Comparing techniques for pterygium surgery. Clin Ophthalmol 2009; 3: 69–74.
McLean C . Pterygium excision with conjunctival autografting. Br J Ophthalmol 1994; 78(5): 421.
Acknowledgements
We thank Esther Holland for her administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The results of eight of the patients included here were presented at EVER 2009 as a free paper abstract.
Rights and permissions
About this article
Cite this article
de Wit, D., Athanasiadis, I., Sharma, A. et al. Sutureless and glue-free conjunctival autograft in pterygium surgery: a case series. Eye 24, 1474–1477 (2010). https://doi.org/10.1038/eye.2010.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.75
Keywords
This article is cited by
-
Comparison of autologous in situ blood coagulum versus sutures for conjunctival autografting after pterygium excision
International Ophthalmology (2014)
-
The influence of pterygium morphology on fibrin glue conjunctival autografting pterygium surgery
International Ophthalmology (2014)
-
Comparison of efficacy of three surgical methods of conjunctival autograft fixation in the treatment of pterygium
International Ophthalmology (2014)