Abstract
Purpose
To report macular thickness values in normal eyes and eyes with diabetic macular edema (DME) using time-domain (TD) and spectral-domain (SD) optical coherence tomography (OCT), and to derive a conversion equation.
Methods
The index study was a prospective investigation conducted on 80 eyes from 40 normal subjects and 130 eyes from 118 patients with DME seen in our clinic. Retinal thickness values from the central 1 mm of the macula and surrounding four ETDRS subfields were acquired using TD-OCT (Stratus OCT) and SD-OCT (SPECTRALIS HRA+OCT). Measurements of the central (C) subfield from both devices were used to derive a conversion equation. The equation was used to predict SD-OCT values using measurements from TD-OCT. Agreement between predicted and actual SD-OCT measurements was assessed.
Results
In normal eyes, the mean difference between TD-OCT and SD-OCT measurements of the C subfield was 76 μm (CI95=74 and 77, respectively). The conversion equation, y=1.029x+72.49, was derived. In eyes with DME, using the equation, SPECTRALIS-predicted values were 5% higher than actual measurements, with 95% of predicted values falling within 9% of the actual measurements. Relocating SD-OCT grids to match the location on TD-OCT resulted in predicted values falling within 7% of actual measurements.
Conclusions
The percent difference between actual thickness measurements from SPECTRALIS and predicted thickness measurements, using the conversion equation, was within reported limits of repeatability of Stratus in eyes with DME. Our equation may help correlate OCT values from both devices in standard care and clinical trials for DME.
Similar content being viewed by others
Introduction
Optical coherence tomography (OCT) generates cross-sectional images of ocular structures by measuring the echo-time delay and intensity of reflected light. It is an excellent non-invasive means of quantitative measurement of retinal thickness and was first described by Huang et al1 in 1991. ‘Zeiss OCT’, introduced in 1996, was the first commercially available OCT device. Zeiss Stratus OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA) became available in 2002.
Stratus OCT is based on a time-domain (TD) detection technique, which depends on a light source and a moving reference mirror. Recent advances in detection techniques have enabled significant increases in OCT imaging speeds. An alternative to TD detection for OCT is a technique known as Fourier or frequency-domain detection.2, 3 Spectral/Fourier-domain OCT (SD-OCT) detects all echoes of light simultaneously. The elimination of the moving reference mirror (present in TD-OCT) allows a dramatic increase in line rate (A-scan rate) in SD-OCT.4, 5, 6 Multiple SD-OCT devices are commercially available; Heidelberg's SPECTRALIS (Heidelberg Engineering Inc., Vista, CA, USA) is one of the commonly used devices in this category. Wolf-Schnurrbusch et al7 compared thickness values from Stratus with five SD-OCT devices. Thickness values from Stratus were significantly lower compared with those from SD-OCT devices. Differences in segmentation algorithm and image acquisition methods lead to different estimates of retinal thickness. SD-OCT is being increasingly preferred over TD-OCT because of the higher acquisition speeds and better resolution of the acquired images.
OCT has shown to be beneficial in the management of eyes with diabetic macular edema (DME). OCT is more sensitive than stereoscopic fundus photography or biomicroscopy for the detection of DME and also allows a more objective assessment of the extent of macular edema.8 Thus, OCT is being increasingly utilized in the diagnosis and treatment of DME. Re-treatment of DME, such as with anti-VEGF agents, in most research protocols is based on central (C) subfield thickness values of ≥250 μm on Stratus OCT. The Diabetic Retinopathy Clinical Research Network (DRCR.net) has been using thickness values from TD-OCT as one of the eligibility criteria and efficacy outcomes across many clinical trials. At least 15 protocols that use Stratus OCT are currently listed on the DRCR.net. These studies have provided tremendous insights into the management of DME. In order to apply the information acquired using Stratus TD-OCT into future clinical practice and follow up patients who were previously scanned on older devices, it is essential to be able to compare thickness measurements from one device with those from the other.
We aim to develop a conversion equation to determine Spectralis SD-OCT equivalents of thickness values measured on Stratus OCT and to determine the accuracy of the derived equation in eyes with DME.
Materials and methods
The index study is a prospective observational investigation designed to evaluate central retinal thickness measurements in normal subjects and in subjects with DME, using TD-OCT and SD-OCT. Institutional review board/ethics committee approval was obtained. The study was conducted in compliance with the Declaration of Helsinki, US Code of Federal Regulations Title-21, and the Harmonized Tripartite Guidelines for Good Clinical Practice (1996). Study subjects gave informed consent before enrollment. The study was conducted at the Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Study population
Subjects without ocular disease and subjects with DME participated in the study. Each subject was scanned on Stratus and Spectralis OCTs on the same day, 10–15 min apart. Additional exclusion criteria included a history of glaucoma or other ocular disease that could affect the retinal thickness measurements. Subjects without ocular diseases were scanned twice on each device. Eyes with DME were scanned once on each device.
TD-OCT
TD-OCT scans were acquired using Stratus OCT. The standard fast macular scan protocol consisting of six radial lines centered on the fovea was used to measure the retinal thickness. A second scan with the same acquisition settings was acquired 5–15 min later for repeatability analysis in eyes without retinal disease by the same operator. The average retinal thickness in the nine standard ETDRS macular grid subfields was calculated using Stratus OCT review software (v.5.0.1) (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Individual scans were reviewed to ensure that scans with a signal-to-noise ratio of ≤3 are not included. Scans with algorithm errors were reviewed and the segmentation lines were manually placed in the correct locations along the inner limiting membrane and at the level of the IS/OS (inner segment/outer segment) junction using the bundled Heidelberg Explorer review software (Heidelberg Engineering Inc.). The average retinal thickness measurements were recorded from the C subfield of both normal and DME groups, and from the surrounding four subfields: superior (S), nasal (N), inferior (I), and temporal (T), of the normal group.
SD-OCT
The SD-OCT scans were acquired using the SPECTRALIS HRA+OCT. Retinal thickness measurements were acquired using a standard 25° × 25° raster scan protocol with a distance of 240 μm between the horizontal scans. The TruTrak function of the Spectralis OCT was utilized to acquire a second scan from eyes without retinal disease with the same acquisition settings as for repeatability analysis. The second scan was acquired by the same operator, 5–15 min after the first one. The average retinal thickness in the nine standard ETDRS macular-grid subfields was calculated using the bundled Heidelberg Explorer software (v.5.3). Individual scans were reviewed to ensure that the segmentation lines are correctly located along the inner limiting membrane and the posterior border of the retinal pigment epithelium (RPE). Scans with algorithm errors were manually corrected using the bundled software. The ETDRS grid was manually placed by trained graders in the Retinal Imaging Research and Reading Center at the Wilmer Eye Institute in order to be centered on the fovea. The average retinal thickness was recorded from the same subfields as the corresponding Stratus OCT scans.
Correction of mismatched locations
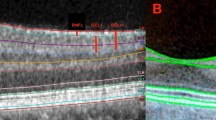
Stratus and the corresponding Spectralis OCT scans of each eye with DME were reviewed to determine whether the ETDRS grids were centered on the same location in both scans. In some scans (n=60), a mismatch was observed between the locations of the ETDRS grid in the Stratus OCT and the corresponding Spectralis OCT. As the Stratus software does not allow moving of the ETDRS grid, the ETDRS grid of the corresponding Spectralis scan was moved to a location that matched the position of the ETDRS grid on the Stratus. The process of matching the location of the ETDRS grid involved coordinating the horizontal scan acquired by the TD-OCT with the respective horizontal scan from the raster acquired by the SD-OCT. The identification process relied on matching the topographic, anatomical, and pathological features in both scans (Figure 1). The middle of the TD-OCT horizontal scan was recognized as the center of the ETDRS grid (for the TD-OCT scan) and a matching point along the previously identified SD-OCT was chosen as the center of the ETDRS grid for the SD-OCT scan.
OCT scans from a subject with DME. The middle section of the figure shows horizontal cuts from Spectralis scan (cut 13/25 of the raster scan) at the level of the anatomical fovea (b), with the green line going through the center of the fovea, Stratus scan (e), with the blue line going through the middle of the scan, and Spectralis scan (cut 14/25 of the raster scan) that matches the Stratus scan on (e), with the green vertical line going through a matching location to that of the blue line in (e). Please note the matching anatomical and pathological features in (e) and (h) that enabled the identification of cut 14 as the Spectralis scan that corresponds to the horizontal scan of Stratus and also enabled the identification of the matching vertical coordinates (blue and green vertical lines in (e) and (h)). The left section of the figure shows the infrared images from Spectralis scan with proper centration of the ETDRS grid on the fovea (a), with the green horizontal line and the center of the grid in (a) corresponding to the horizontal cut and the vertical green line in (b), Stratus scan (d), and Spectralis after moving the ETDRS grid to a location that matches the grid in (d) (g). The right section of the figure shows the thickness maps in false colors from Spectralis OCT scan with proper centration of the ETDRS grid on the fovea (c), Stratus OCT scan (f), and Spectralis after moving the ETDRS grid to matching locations (i). In this patient, the average macular thickness in C was 548 μm when measured by Stratus OCT. According to the equation, the predicted Spectralis OCT value is 636 μm, which represents 115% of the measured values (555). After matching the locations of the ETDRS grid, as shown in this figure, the Spectralis OCT measurement of C was 593 μm, which lowered the ratio between predicted and actual spectralis measurements to 1.07 (107%).
Statistical analysis
The acquired measurements were recorded and analyzed using the IBM Statistical Package for the Social Sciences (IBM SPSS Inc., Chicago, IL, USA), release 19.0.0.
A normative reference range was calculated for each technology, using the data acquired from normal subjects, in each of the five C subfields: C, S, N, I, and T. The second scan acquired in normal subjects was used to analyze the repeatability of each device in the five C subfields, indicated by the coefficient of repeatability as described by Bland–Altman. The measurements from the C subfield from each device were fitted into a regression analysis curve and the linear correlation between the measurements was estimated. A conversion equation was derived from the linear relationship of the C subfields. Mixed effects model was used to correct for the correlation between eyes from the same patient.
The conversion equation was then employed to estimate the SD-OCT values in patients with DME based on the measurements from TD-OCT. An analysis of the level of agreement, as described by Bland–Altman, between the predicted SD-OCT values and the actual SD-OCT measurements was conducted. A secondary analysis of the agreement was conducted after moving the SD-OCT grids to match the location on the TD-OCT.
Results
A total of 40 subjects (80 eyes) without retinal diseases (mean age: 35±10 years) and 118 patients (130 eyes) with DME (mean age: 63±9 years) were enrolled in the study. The demographics of the study subjects are shown in Table 1.
Normal eyes
The mean thickness of the C subfield was 188 μm (SD ±20 μm) in eyes without retinal disease when measured by TD-OCT and 266 μm (SD ±21 μm) on SD-OCT, with an average difference of 76 μm (CI95=74, 77). The mean thickness in the subfields N, S, T, and I was: 266, 268, 255, and 267 μm, respectively, when measured by TD-OCT, and 340, 340, 327, and 336 μm, respectively, when measured by SD-OCT (Table 2). The difference in average thickness as measured by both OCT technologies was statistically significant in all subfields (P<0.01).
Coefficient of repeatability of retinal thickness in the C subfield was 17 μm for TD-OCT and 5 μm for SD-OCT. TD-OCT measurements of retinal thickness in normal subjects were highly correlated with the corresponding SD-OCT measurements in all subfields (Table 2), with Pearson correlation coefficient (r)=0.98 in the C subfield. The TD-OCT and SD-OCT measurements from the C subfields were fitted into a regression curve best described by a linear relationship with an estimated slope of 1.029 and estimated intercept of 72.49 (Figure 2).
Eyes with DME
The mean thickness of the C subfield in eyes with DME was 426 μm (SD ±130 μm) when measured by TD-OCT, and 487 μm (SD ±127 μm) when measured by SD-OCT. The measurements from both technologies were highly correlated (r=0.99), with an average difference of 61 μm (CI95=57, 64) (Table 3).
TD-OCT measurements of the C subfield were used to predict the SD-OCT values by employing the conversion equation derived from thickness measurements in normal eyes; y=[1.029x]+72.49, where y is the predicted SD-OCT value and x is the average thickness in the C subfields as measured by TD-OCT. The equation using mixed effects model was y=[1.029*x]+72.34. As the difference between the two equations was almost negligible, we used the former equation for all the analyses in the index study. The predicted SD-OCT values were highly correlated with the measured values (r=0.99). The mean predicted thickness (511 μm) was higher than the mean of the actual measured thickness values (487 μm) (P<0.001), with mean difference between measured and predicted values of 24 μm (CI95=20, 28) (Table 3).
The difference between predicted and actual values in the C subfield increased with the increase in average thickness (r2=0.17). The mean of the predicted values was 5% higher than the mean of actual measurements, with 95% of the actual measurements falling within 9% of the predicted values. Overall, 7 out of the 130 predicted values were ≥9% away from the actual values. Bland–Altman plot (Figures 3a and c) depicts the level of agreement between actual and predicted SD-OCT values.
Bland–Altman plots showing the agreement between the predicted Spectralis values in the C subfield (c) and the Spectralis measurements in (c) before (a, c) and after (b, d) matching the locations of the ETDRS grids of both Stratus and Spectralis scans. Matching the locations of the grids resulted in smaller standard deviations (SD) and hence narrower 95% confidence intervals. When the absolute measurements in microns were used (a, b), the predicted values tended to increase with the increase in thickness, as indicated by the linear fit line. Such tendency could be corrected for by using the ratio between predicted and actual Spectralis measurements instead of the absolute values (c, d), as indicated by the linear fit lines.
After moving the ETDRS grid of the SD-OCT to a matching location of the TD-OCT grid (60 eyes), the predicted SD-OCT values remained highly correlated to the measured TD-OCT values (r=0.99), with a mean thickness of 489 μm (SD±127 μm). The difference between the mean SD-OCT measurements of the C subfield, before and after moving the grid, was not statistically significant (P=0.28), with a mean difference of 1.5 μm (CI95=−1.3, 4.3) (Table 3).
Moving the grid to a matched location resulted in slight improvement of the levels of agreement between the predicted values and actual SD-OCT measurements of the C subfield to a mean difference of 23 μm (CI95=20, 25). The difference between predicted and actual values in the C subfield, after moving the grid, increased with the increase in average thickness (r2=0.17).
The predicted values were still higher, after moving the grid, than the actual measurements, with an average difference of 4.6%. However, 95% of the actual measurements fell within 6.2% of the predicted values; all predicted measurements using the conversion equation fell within 7% of the actual values. Bland–Altman plot (Figures 3b and d) depicts the level of agreement between actual measurements after matching locations and predicted SD-OCT values.
Discussion
SD-OCT is likely to be used increasingly in standard practice as well as in clinical trials and research. The average C subfield (CSDOCT.Spectralis) thickness for the eyes without retinal diseases in our study was 266 μm, which is similar to the mean CSDOCT of 270 μm reported by Grover et al9 in their measurement of thickness values using the same device. The retinal thickness measurements from Stratus and Spectralis showed an average difference of 76 μm. The difference in measurements between both devices is in part because of the different segmentation algorithms of the two technologies. Stratus TD-OCT measures retinal thickness from IS/OS to ILM. On the other hand, each SD-OCT device has a different algorithm; the Spectralis SD-OCT used in our study measures retinal thickness between the ILM and the posterior border of RPE.
Correlation of thickness measurements between Stratus and various SD-OCT devices has been reported to be high, with correlation coefficients of 0.8–0.9 for Stratus and Spectralis OCT measurements in normal eyes.10, 11 With an observed correlation coefficient of 0.98 in our study, our results are consistent with the previous reports.
Despite the high correlation coefficients, poor agreement in thickness measurements between Stratus and SD-OCT devices has been reported. Leung et al12 reported the 95% limits of agreement between Stratus and 3D-OCT to be 3.9–37.8 μm. Such poor agreements are considered too large to allow devices to be used interchangeably.12, 13, 14
Development of conversion equations has been suggested to allow follow-up of patients previously scanned on Stratus OCT and to enable interchangeable usage of both types of OCT. Forte et al15suggested a correction factor of 1.1 when converting values from Stratus to equate values from SD-SLO/OCT. Conversion equations are unlikely to yield perfect agreement between actual and predicted measurements. Factors other than segmentation algorithm (for example, density of sections, acquisition method, and acquisition speed) may contribute to differences in thickness measurements among devices. In this study, we have derived a conversion equation using thickness values from the central 1 mm of the retina from 80 normal eyes: y=(1.029x)+72.49. When we applied this equation in eyes with DME, 95% of the actual measurements fell within 9% of the values predicted by our derived equation. Such finding is within the limits of repeatability of the C subfield thickness measurements of Stratus, which has been reported to be 11%.16 In fact, 98% of the predicted values in our study were within the repeatability limits of Stratus reported in 212 eyes with DME.16 Although not the ideal solution, conversion equations are important in selected cases to compare thickness measurements in clinical practice, especially among patients who were previously followed on Stratus OCT and are now changing to SD-OCT.
In our study, a number of Stratus scans (n=60; 46%) were not centered on the anatomical fovea, most likely because of the relatively poor fixation in patients with DME. Contrary to Stratus, Spectralis grids can be manually centered on the anatomic fovea after image acquisition and before the average measurements are recorded. Such mismatch in the grid location introduced a measure of variability in the readings. As moving the Stratus grid was not possible, because of limitations on the reviewing software, and would not have been scientifically acceptable, scans were reviewed for a second time and the Spectralis grids of the mismatched pairs were moved on to match the location on Stratus, yielding a new Spectralis thickness in the process. Although relocating the grid did not result in a statistically significant change in thickness measurement, it helped to eliminate six of the seven previously identified outliers, resulting in all predicted values falling within 7% of the actual measurements. We recommend that if clinician scientists wish to improve the accuracy of the equation, they should compare the anatomic location of the ETDRS grid on Stratus with the location on Spectralis.
Lammer et al17 compared thickness measurements from Stratus OCT with measurements from three different SD-OCT devices for 30 eyes with DME. Comparison with Spectralis OCT showed the CI95 of difference in thickness to be 62–93 μm. In our study, the CI95 for C subfield was 57–64 and 60–65 μm, before and after relocating the grid, respectively. Figures 3a and b show that difference in retinal thickness increases with the increase in retinal thickness measurements. However, the fact that 95% of the actual measurements fell within 9% of the predicted values illustrates that the percentage difference remains constant (Figures 3c and d).
Differences in thickness values among OCTs may differ depending on the specific retinal pathology.18 Our equation was derived from eyes without retinal diseases and we have determined its accuracy in predicting Spectralis thickness values in eyes with DME. However, it is possible that our equation will not work in retinal diseases other than DME.
We believe that the intercept in our equation (72.49 m) can be largely explained by the differences in the measured tissue between both devices, where Spectralis includes the RPE layer and outer segments of the photoreceptors in the measured thickness. We also believe that the slope in our equation largely represents the differences in interpolation algorithms and scanning protocols between both devices.
Different SD-OCT devices have slightly different acquisition protocols and interpolation and segmentation algorithms. Hence, average thickness measurement is expected to be different for different SD-OCT devices. Lammer et al reported the average difference in thickness measurements between Cirrus and Spectralis SD-OCT to be 19 μm, and between Spectralis and 3D OCT to be 55 μm. We cannot be certain that the accuracy of our equation will be maintained when comparing thickness measurements from Stratus TD-OCT with SD-OCT devices other than Spectralis.17
In addition, the derived conversion equation may not be applicable for points away from the center. The scan acquisition protocol of Stratus OCT is such that as one moves further away from the intersection, the scanned lines move further away from each other. Therefore, there is more interpolation and estimation of the intervening retinal thickness in Stratus compared with Spectralis OCT, where raster scans allow for consistent interpolation across the scan regardless of the location of the grid. Hence, the same equation may not apply to other subfields.
A Stratus OCT thickness of ≥250 μm is one of the eligibility criteria for enrolling a patient into many DME clinical trials and an important criterion for re-treatment in many study protocols. According to our equation, the predicted thickness on Spectralis would be 330 μm. The predicted thickness measurements in our study were, on average, 5% higher than the actual thickness measurements. Hence, the actual thickness equivalent on Spectralis for a Stratus value of 250 μm is most likely ∼314 μm.
The equation also provides a mechanism to compare the results from studies that have used Stratus TD-OCT with studies that have employed or will employ Spectralis SD-OCT. The methodology used in our index study may be duplicated or further extended to investigate the correlation among other OCT devices and in different retinal diseases. Such studies are indicated, given the evolving advancements in OCT technology and the protean utilities of OCT in clinical care and research.

References
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W et al. Optical coherence tomography. Science 1991; 254 (5035): 1178–1181.
Golubovic B, Bouma BE, Tearney GJ, Fujimoto JG . Optical frequency-domain reflectometry using rapid wavelength tuning of a Cr(4+):forsterite laser. Opt Lett 1997; 22 (22): 1704–1706.
Lexer F, Hitzenberger CK, Fercher AF, Kulhavy M . Wavelength-tuning interferometry of intraocular distances. Appl Opt 1997; 36 (25): 6548–6553.
Choma MA, Sarunic MV, Yang CH, Izatt JA . Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express 2003; 11 (18): 2183–2189.
de Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE . Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett 2003; 28 (21): 2067–2069.
Leitgeb R, Hitzenberger CK, Fercher AF . Performance of Fourier domain vs. time domain optical coherence tomography. Opt Express 2003; 11 (8): 889–894.
Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 2009; 50 (7): 3432–3437.
Browning DJ, McOwen MD, Bowen Jr RM, O’Marah TL . Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology 2004; 111 (4): 712–715.
Grover S, Murthy RK, Brar VS, Chalam KV . Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am J Ophthalmol 2009; 148 (2): 266–271.
Grover S, Murthy R, Brar V, Chalam K . Comparison of retinal thickness in normal eyes using Stratus and Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci 2010; 51 (5): 2644.
Carpineto P, Nubile M, Toto L, Aharrh Gnama A, Marcucci L, Mastropasqua L et al. Correlation in Foveal thickness measurements between spectral-domain and time-domain optical coherence tomography in normal individuals. Eye 2009; 24 (2): 251–258.
Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, Pang CP et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2008; 49 (11): 4893–4897.
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT . Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci 2008; 49 (10): 4290–4296.
Kiernan DF, Hariprasad SM, Chin EK, Kiernan CL, Rago J, Mieler WF . Prospective comparison of cirrus and stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol 2009; 147 (2): 267–275 e2.
Forte R, Cennamo GL, Finelli ML, de Crecchio G . Comparison of time domain Stratus OCT and spectral domain SLO/OCT for assessment of macular thickness and volume. Eye 2008; 23 (11): 2071–2078.
Krzystolik MG, Strauber SF, Aiello LP, Beck RW, Berger BB, Bressler NM et al. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology 2007; 114 (8): 1520–1525.
Lammer J, Scholda C, Prunte C, Benesch T, Schmidt-Erfurth U, Bolz M . Retinal thickness and volume measurements in diabetic macular edema: a comparison of four optical coherence tomography systems. Retina 2011; 31 (1): 48–55.
Han IC, Jaffe GJ . Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am J Ophthalmol 2009; 147 (5): 847–858, 58 e1.
Acknowledgements
We would like to acknowledge Jiangxia Wang from the Biostatistics Clinic at the Dana Center for Preventive Ophthalmology, Johns Hopkins University for her help with the statistical analysis in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
QDN is the recipient of the Physician Scientist Award from the Research to Prevent Blindness and the RO1 Award from the National Eye Institute, National Institutes of Health (EY17577). QDN, DVD, and the Johns Hopkins University have received research support from Heidelberg Engineering, Inc. QDN has served on the Scientific Advisory Board for Heidelberg, Inc. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ibrahim, M., Sepah, Y., Symons, R. et al. Spectral- and time-domain optical coherence tomography measurements of macular thickness in normal eyes and in eyes with diabetic macular edema. Eye 26, 454–462 (2012). https://doi.org/10.1038/eye.2011.293
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.293
Keywords
This article is cited by
-
Phosphorylation of cyclophilin D at serine 191 regulates mitochondrial permeability transition pore opening and cell death after ischemia-reperfusion
Cell Death & Disease (2020)
-
Primary outcomes of the VIDI study: phase 2, double-masked, randomized, active-controlled study of ASP8232 for diabetic macular edema
International Journal of Retina and Vitreous (2019)
-
Incidence and risk factors for neovascular age-related macular degeneration in the fellow eye
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
-
Selective and complementary use of Optical Coherence Tomography and Fluorescein Angiography in retinal practice
Eye and Vision (2016)
-
Macular ischemia and outcome of vitrectomy for diabetic macular edema
Japanese Journal of Ophthalmology (2015)