Abstract
Background/aim
The aim of this study is to report a reduction in the thickness of the ganglion cell complex (GCC) after vitrectomy with internal limiting membrane (ILM) peeling in eyes with idiopathic macular hole (MH).
Methods
Twenty-eight consecutive eyes with an idiopathic MH treated by vitrectomy with ILM peeling were studied. All eyes had an intravitreal injection of indocyanine green to make the ILM more visible. The best-corrected visual acuity (BCVA), GCC thickness measured by spectral domain optical coherence tomography, and retinal sensitivity measured by microperimetry were determined before and at 3 and 6 months after the vitrectomy.
Results
The MH in all eyes was closed after the initial surgery. The BCVA was significantly improved at 3 and 6 months (P<0.001 and P<0.001, respectively). The thickness of the GCC was significantly reduced at 3 and 6 months postoperatively (P<0.001 and P<0.001, respectively). The GCC thickness was significantly correlated with the retinal sensitivity in the central 10 degrees at 6 months (r=0.55, P=0.004).
Conclusion
A reduction of the GCC thickness was observed after vitrectomy with ILM peeling for idiopathic MH.
Similar content being viewed by others
Introduction
The rate of anatomical closure of idiopathic macular holes (MHs) improved significantly after the internal limiting membrane (ILM) was removed during the vitrectomy.1 Staining the ILM with indocyanine green (ICG) has been a standard technique during its removal around the MH, and currently, other dyes such as Brilliant blue G are used for this because of the reported cytotoxicity of ICG. A recent prospective randomized study showed a significant improvement in the rate of primary MH closure with lower incidence of reoperation in the ILM-peeled group.2
The development of spectral domain optical coherence tomography (SD-OCT) has made it possible to study the morphology of the different retinal layers in more detail.3 Relevant to this study, the ganglion cell complex (GCC), the layer between the ILM and inner nuclear layer of the retina, can be easily differentiated and its thickness measured by a program embedded in the SD-OCT. The functioning of the inner retinal layers is related to the thickness of the GCC, and the thickness has been found to be reduced in glaucoma patients.4
We have reported that the recovery of vision after MH surgery was well correlated with the presence of the photoreceptor inner/outer segment (IS/OS) line, the external limiting membrane (ELM), and normal foveal morphology in the OCT images.5, 6, 7 Some of the cases also have a thinning of the GCC, although there has not been a study that reported a reduction in the GCC thickness after MH surgery with ILM peeling. There have been studies showing that the retinal nerve fiber layer changes after idiopathic MH surgery.8, 9, 10
Thus, the purpose of this study was to determine whether the GCC becomes thinner after ILM peeling for an MH. The relationship between retinal sensitivity and the GCC thickness was also determined.
Patients and methods
This was a retrospective and non-randomized study of 28 eyes of 28 consecutive patients with a MH. All cases were examined and treated at the Chiba University Hospital from December 2009 to April 2010. This study was approved by the Institutional Review Board of Chiba University Graduate School of Medicine, and the procedures used conformed to the tenets of the Declaration of Helsinki. Patients were informed on the purpose of the treatments and possible complications, and a written informed consent was obtained from all patients.
The surgical procedure consisted of 23-gauge, 3-port pars plana vitrectomy combined with phacoemulsification and aspiration, and implantation of a foldable intraocular lens. The surgery was performed by three of the authors (TB, ES, SY). As all of the surgeons were experienced and had performed many MH surgeries with ILM peeling, an improvement of the surgeons’ skill during this time period can be eliminated. These three surgeons used similar techniques, and the surgical times were not significantly different among them.
A posterior vitreous detachment was created if one was not present. Cases with an obvious epiretinal membrane (ERM) were excluded to avoid the effect of ERM peeling. Cases with dense cataract (grade 3 or more, by Emery–Little scale) were also excluded to eliminate the possibility that the postoperative visual improvement was due to the cataract extraction.
The ILM temporal to the macular was made visible by ICG, and it was lifted with a 23-gauge needle and grasped with an ILM forceps. The ILM was peeled off the retina over approximately three disc diameters in all cases. ICG was prepared as 0.125% solution diluted in balanced salt solution and was washed out immediately after the injection. In all cases, an air tamponade was used, and patients were instructed to maintain a prone position for at least 3 days after the surgery.
All patients had a complete ophthalmic examination, including measurements of the best-corrected visual acuity (BCVA), slit-lamp examination, indirect ophthalmoscopy, microperimetry (MP1, Nidek Technologies, Aichi, Japan), and SD-OCT (RTVue-100, Optovue, Fremont, CA, USA). These tests were done at the initial examination and at 3 and 6 months postoperatively.
The retinal sensitivity was determined with the MP1 with the software (version, 1.4.2.SP1) embedded in the device. The Goldmann III, 4−2 staircase strategy was used and the sensitivity of 24 locations within the central 10 degrees was determined.11
The GCC thickness was determined by the GCC measuring mode of the original software of the SD-OCT. This program automatically measures the total thickness of the nerve fiber layer, ganglion cell layer, and inner plexiform layer (IPL). The GCC thickness was measured within a 6 mm × 6 mm square centered slightly temporal to the fovea, and the values are given by the average of the overall area except for the fovea. As the ganglion cell complex within 0.75 mm of the foveal center is too thin to be reliably measured in the region, this area was excluded from the GCC analysis. The reproducibility of the measurement of GCC was confirmed by multiple observations of the studied and fellow eyes. The baseline and postoperative GCC thickness was also compared with that in unaffected eyes, which had no ocular pathology, including MH, ERM, and glaucoma.
Statistical analyses for the changes in the BCVA and GCC thickness were made using paired t-tests. The relationships between the GCC and the retinal sensitivity were tested by Spearman’s coefficients of correlation. P-values <0.05 were considered to be statistically significant.
Results
The patients’ age ranged from 54 to 77 (mean 65.7±7.3) years. Nine men and 19 women were studied. The estimated duration from onset to surgery ranged 1–8 months with a mean of 3.6±1.7 months. The MH stage was stage 2 in 7 eyes, stage 3 in 12 eyes, and stage 4 in 9 eyes. The MH size was calculated to be the average of the vertical and horizontal diameters, and it was 801±201 μm. The baseline mean BCVA was 0.82±0.31 logarithm of the minimum angle of resolution (logMAR) units (0.15 in decimal units) with a range from 0.16 to 1.22 logMAR units (0.05 to 0.7).
The MH was closed in all cases after the initial surgery. No intraoperative or postoperative complications including an elevation of intraocular pressure were observed. The findings in a representative case are presented in Figure 1. The mean preoperative BCVA was 0.82±0.31 logMAR units (0.15), and it was 0.49±0.28 logMAR units (0.32) at 3 months and 0.37±0.27 logMAR units (0.43) at 6 months (Figure 2). The improvement in the BCVA from the baseline was significant at each time (P<0.001 at 3 months and P<0.001 at 6 months).
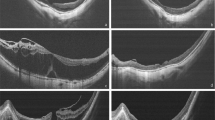
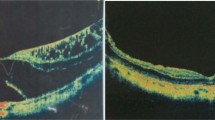
Left eye of 62-year-old Japanese woman treated by pars plana vitrectomy with ILM peeling using ICG. Two months after the onset of vision decrease, the patient underwent vitrectomy. A stage 3 MH was found (a) and the preoperative decimal BCVA was 0.08. The average retinal sensitivity of the central 10 degrees measured by MP1 microperimetry was 11.2 dB (b). GCC thickness map was determined by SD-OCT, and the average thickness was 103.2 μm (c). Three months after the surgery, the MH was closed and the decimal BCVA improved to 0.2 (d). Retinal sensitivity of the central 10 degrees was 16.5 dB (e). The average GCC thickness decreased to 79.2 μm (f). The area shown in red indicates significantly thinner GCC (P<0.01). The retinal sensitivity in the area with GCC thinning temporal and inferior to fovea is lower than other areas. Six months after the surgery, the MH is closed and the decimal BCVA was improved to 0.4 (g). Retinal sensitivity of the central 10 degrees is 14.8 dB (h). The average GCC thickness decreased to 76.6 μm (i). The area with the GCC thinning temporal to the fovea has enlarged. The GCC thickness is shown between two white lines in a preoperative horizontal cross-sectional OCT image (j). The closure of MH was observed and the GCC thickness was slightly reduced at 3 months after the surgery (k). At 6 months after surgery, the GCC was thinner especially at temporal area (l).
The mean GCC thickness at 3 and 6 months after the surgery was significantly reduced from 95.5±6.8 to 84.9±10.0 and to 84.2±10.8 μm (P<0.001 and P<0.001, respectively).
The changes in the GCC thickness from the baseline to 6 months after the surgery are shown in Figure 3. The mean thickness of GCC in the unaffected fellow eyes was 94.7±5.4 μm, and this value was used as the normal GCC thickness in this study. The postoperative GCC thickness was significantly thinner than that of the fellow eyes at 3 and 6 months postoperatively (P=0.007 and P=0.010, respectively). The thinning of the GCC was usually located temporal and inferior to the fovea where the initial ILM flap was made. However, the area of GCC thinning was larger than the area where the retina was touched by the forceps (Figure 1). This was confirmed by reviewing a video in each case. The algorithm for the GCC measurements by RTVue-100 correctly followed the retinal surface and outer edge of the IPL even after ILM peeling. The accuracy of the measurements was confirmed in all GCC measurements around the fovea by viewing the raw data.
Changes of GCC thickness from baseline to 6 months after the MH surgery. The GCC thickness was reduced in all cases at 6 months after surgery (P<0.001). In the studied eyes, the GCC thickness at 6 months was smaller than normal GCC thickness of 94.7 μm, except for three eyes. The normal GCC thickness is marked by the shadowed area (94.7±5.4 μm) based on the GCC thickness in unaffected fellow eyes. Broken lines indicate the average thickness of GCC in fellow eyes.
At 6 months, the GCC thickness and retinal sensitivity of the central 10 degrees was moderately correlated (r=0.55; P=0.004; Figure 4).
Discussion
Our results showed that all of the idiopathic MHs were closed after vitrectomy with the ILM made visible by ICG. The BCVA improved significantly in all eyes, and the GCC thickness was significantly reduced at 3 and 6 months. In addition, there was a significant correlation between the GCC thickness and the retinal sensitivity in the central 10 degrees at 6 months.
The GCC thickness measurements were originally designed to evaluate the ganglion cell loss in the macular area where 50% of all ganglion cells exist in glaucoma cases. The GCC has been shown to be significantly thinner in advanced glaucoma patients than in mild glaucoma cases.4 The SD-OCT scan covers a 6 mm × 6 mm area and is centered slightly temporal to the fovea to detect early ganglion cell loss in eyes with glaucoma. As this program does not include the area within 0.75 mm of the foveal center (1.5-mm diameter circle) for GCC measurement, the thickened retina around the MH preoperatively had little influence on preoperative GCC thickness. This OCT-scanned area corresponds to 20 degrees, which includes the central 10 degrees measured by MP1 microperimetry. This overlap allowed us to determine the relationship between these two values. In addition, this area covered the area where the ILM was peeled, and it was possible to study the functional and morphological changes of this area after ILM peeling.
According to previous reports, the thickness of GCC measured by RTvue-100 in normal eyes ranged from 93.7 to 95.1 μm.4, 12, 13, 14 The GCC thickness obtained from the unaffected fellow eyes was 94.7±5.4 μm, which is comparable to that in other studies. The postoperative GCC thickness in our study was 84.2±10.8 μm at 6 months, and it was thinner than normal GCC thickness by approximately 10 μm. The reduction of GCC thickness was significant after ILM peeling for MH surgery. We observed a greater reduction of the GCC thickness at 6 months than at 3 months. This might indicate an ongoing process of resolution of retinal edema caused by ILM peeling. Further studies with longer observation periods would be helpful in confirming this hypothesis.
We suggest two possible explanations for the GCC reduction. The first is a mechanical manipulation of the ILM, which damaged the GCC. The thickness of ILM has been reported to be about 2.5 μm in the posterior pole,15 and the removal of the ILM means that the reduction of GCC thickness should be by at least this much. The presence of neuronal and ganglion cells on surgically excised ILM by immunohistochemistry16 supports this idea. The second possibility is that the reduction was due to the cytotoxicity of ICG used during the ILM peeling. There are in vivo and in vitro studies demonstrating the cytotoxic effects of ICG on ganglion cells.17, 18, 19, 20 There have been reports that intravitreal ICG remains more than 3 months after vitrectomy,21, 22, 23 and we studied GCC thickness at 3 and 6 months in this study. To confirm the cytotoxic effects of ICG, we need to study a group of cases treated with ILM peeling without the use of a dye. However, it is quite difficult to confirm the area of ILM peeling without making the ILM visible, which is critical for comparing the postoperative data.
There was a significant correlation between the GCC thickness and the retinal sensitivity in the central 10 degrees at 3 and 6 months. A thinner GCC was associated with lower retinal sensitivity as shown earlier in glaucoma cases.24, 25 As the GCC thickness changed in the area corresponding to the ILM peeled area, measuring not only central visual acuity but retinal sensitivity seems important to evaluate postoperative macular function after ILM peeling.
The postoperative BCVA improved significantly in spite of the GCC thinning. The foveal microstructure, for example, MH closure and IS/OS and ELM restoration, must have more influence on the recovery of central visual acuity. However, the GCC thickness affected retinal sensitivity surrounding the fovea, because the ganglion cells are absent at the foveal pit and the IS/OS line, and the ELM are usually well restored outside the fovea.
It has not been reported that the temporal retina is thinner after ERM removal. The retina is usually thicker because of traction by the ERM, and it takes some time to reduce the swelling after the surgery. In many ERM cases, the retina remains thickened and the foveal depression is shallow for years. On the other hand, our MH cases had a GCC thinning postoperatively. MH cases usually have no retinal thickening, except for the area adjacent to MH. This difference can explain the lack of thinning of the temporal retina in postoperative ERM cases.
The limitations in this study are the short observation period of 6 months, small number of eyes, no control eyes for use of ICG, and its retrospective nature. Therefore, further studies with longer observation periods and larger sample size are needed to confirm these results. However, the thinning of GCC was significant even at 3 months, and we believed that it was meaningful to report these data at this time as the preliminary data.
In conclusion, we investigated the reduction of GCC thickness after ILM peeling using ICG. Although the ILM peeling is essential for successful MH surgery, a significant correlation between the postoperative thickness of the GCC and retinal sensitivity suggested the importance of a postoperative change of GCC thickness.

References
Kadonosono K, Itoh N, Uchio E, Nakamura S, Ohno S . Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol 2000; 118 (8): 1116–1118.
Lois N, Burr J, Norrie J, Vale L, Cook J, McDonald A et al. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci 2011; 52 (3): 1586–1592.
Mujat M, Chan R, Cense B, Park B, Joo C, Akkin T et al. Retinal nerve fiber layer thickness map determined from optical coherence tomography images. Opt Express 2005; 13 (23): 9480–9491.
Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 2009; 116 (12): 2305–2314 e2301–2302.
Baba T, Yamamoto S, Arai M, Arai E, Sugawara T, Mitamura Y et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina 2008; 28 (3): 453–458.
Ooka E, Mitamura Y, Baba T, Kitahashi M, Oshitari T, Yamamoto S . Foveal microstructure on spectral-domain optical coherence tomographic images and visual function after macular hole surgery. Am J Ophthalmol 2011; 152 2: 283–290 e281.
Miura G, Mizunoya S, Arai M, Hayashi M, Yamamoto S . Early postoperative macular morphology and functional outcomes after successful macular hole surgery. Retina 2007; 27 (2): 165–168.
Brazitikos PD, Katsimpris JM, Tsironi E, Androudi S . Retinal nerve fiber layer thickness evaluation after trypan blue-assisted macular surgery. Retina 2010; 30 (4): 640–647.
Mitamura Y, Suzuki T, Kinoshita T, Miyano N, Tashimo A, Ohtsuka K . Optical coherence tomographic findings of dissociated optic nerve fiber layer appearance. Am J Ophthalmol 2004; 137 (6): 1155–1156.
Yamashita T, Uemura A, Kita H, Sakamoto T . Analysis of the retinal nerve fiber layer after indocyanine green-assisted vitrectomy for idiopathic macular holes. Ophthalmology 2006; 113 (2): 280–284.
Okada K, Kubota-Taniai M, Kitahashi M, Baba T, Mitamura Y, Yamamoto S . Changes in visual function and thickness of macula after photodynamic therapy for age-related macular degeneration. Clin Ophthalmol 2009; 3: 483–488.
Schulze A, Lamparter J, Pfeiffer N, Berisha F, Schmidtmann I, Hoffmann EM . Diagnostic ability of retinal ganglion cell complex, retinal nerve fiber layer, and optic nerve head measurements by Fourier-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2011; 249 (7): 1039–1045.
Kim NR, Lee ES, Seong GJ, Kang SY, Kim JH, Hong S et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol 2011; 95 (8): 1115–1121.
Kim NR, Kim JH, Lee J, Lee ES, Seong GJ, Kim CY . Determinants of perimacular inner retinal layer thickness in normal eyes measured by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52 (6): 3413–3418.
Foos RY . Vitreoretinal juncture; topographical variations. Invest Ophthalmol 1972; 11 (10): 801–808.
La Heij EC, Dieudonne SC, Mooy CM, Diederen RM, Liem AT, van Suylen RJ et al. Immunohistochemical analysis of the internal limiting membrane peeled with infracyanine green. Am J Ophthalmol 2005; 140 (6): 1123–1125.
Iriyama A, Uchida S, Yanagi Y, Tamaki Y, Inoue Y, Matsuura K et al. Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci 2004; 45 (3): 943–947.
Yip HK, Lai TY, So KF, Kwok AK . Retinal ganglion cells toxicity caused by photosensitising effects of intravitreal indocyanine green with illumination in rat eyes. Br J Ophthalmol 2006; 90 (1): 99–102.
Balaiya S, Brar VS, Murthy RK, Chalam K . Effects of indocyanine green on cultured retinal ganglion cells in-vitro. BMC Res Notes 2009; 2: 236.
Murata M, Shimizu S, Horiuchi S, Sato S . The effect of indocyanine green on cultured retinal glial cells. Retina 2005; 25 (1): 75–80.
Sayanagi K, Ikuno Y, Soga K, Sawa M, Oshima Y, Kamei M et al. Residual indocyanine green fluorescence pattern after vitrectomy for idiopathic macular hole with internal limiting membrane peeling. Br J Ophthalmol 2007; 91 (7): 939–944.
Ciardella AP, Schiff W, Barile G, Vidne O, Sparrow J, Langton K et al. Persistent indocyanine green fluorescence after vitrectomy for macular hole. Am J Ophthalmol 2003; 136 (1): 174–177.
Tadayoni R, Paques M, Girmens JF, Massin P, Gaudric A . Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology 2003; 110 (3): 604–608.
Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY . Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci 2010; 51 (9): 4646–4651.
Cho JW, Sung KR, Lee S, Yun SC, Kang SY, Choi J et al. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2010; 51 (12): 6401–6407.
Acknowledgements
We thank Professor Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami for his critical discussion and editing the final manuscript. This work was supported by the Japan Society for the Promotion of Science KAKENHI 23791966, Grant-in-Aid for Young Scientists (B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Baba, T., Yamamoto, S., Kimoto, R. et al. Reduction of thickness of ganglion cell complex after internal limiting membrane peeling during vitrectomy for idiopathic macular hole. Eye 26, 1173–1180 (2012). https://doi.org/10.1038/eye.2012.170
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.170
Keywords
This article is cited by
-
Outcomes after Epiretinal Membrane Surgery with or Without Internal Limiting Membrane Peeling
Ophthalmology and Therapy (2019)
-
Characteristics of retinal vessels in surgically closed macular hole: an optical coherence tomography angiography study
Graefe's Archive for Clinical and Experimental Ophthalmology (2017)
-
Inner retinal thinning after Brilliant Blue G-assisted internal limiting membrane peeling for vitreoretinal interface disorders
International Ophthalmology (2017)
-
Analysis of the ganglion cell layer and photoreceptor layer using optical coherence tomography after idiopathic epiretinal membrane surgery
Graefe's Archive for Clinical and Experimental Ophthalmology (2015)
-
Brilliant Blue G assisted Epiretinal Membrane Surgery
Scientific Reports (2014)