Abstract
Purpose
To assess the safety and efficacy of a single session of subthreshold micropulse (SM) yellow laser (577 nm) in the treatment of chronic central serous chorioretinopathy (CSCR).
Methods
This was a retrospective analysis of 15 eyes of 13 patients with CSCR of >3 months duration who had been treated with SM yellow laser (577 nm). All patients had been treated using multiple spots of laser with a duty cycle of 10% over areas of focal and diffuse leak, as seen on fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA). Reduction in subretinal fluid height on spectral domain optical coherence tomography (SD-OCT) was used to measure the response to treatment.
Results
The mean follow-up was at 8 weeks (4–19 weeks). All eyes responded to treatment. The mean subretinal fluid height pre and post treatment was 232 and 49 μm, respectively, showing a 79% average reduction (P<0.001) in fluid height. There was no evidence of retinal pigment epithelium or retinal damage on SD-OCT, FFA, or fundus autofluorescence. Median visual improvement was one line on Snellen’s visual acuity chart (P=0.015). Microperimetry was performed in eight eyes of which six eyes (75%) showed an improvement in the threshold values post treatment.
Conclusion
SM yellow laser is an effective treatment option for chronic CSCR.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSCR) is characterized by an idiopathic serous detachment of the neurosensory retina secondary to defects in the retinal pigment epithelium (RPE). The etiology and pathophysiology of CSCR is varied but ultimately leads to widespread hyperdynamic and hyperpermeable choroidal circulation with localized areas of choroidal nonperfusion.1, 2
CSCR is usually self-limiting with good recovery of visual function. However, 33–50% of cases can have recurrences with long-standing areas of neurosensory detachment leading to permanent visual impairment.3, 4, 5 The definition of the duration of chronic CSCR is inconsistent; some studies have used 3 months6 and some studies have used 6 months,7 we have used 3 months in our study. Treatment is indicated in patients requiring faster recovery of vision or in those with permanent visual impairment secondary to CSCR in the fellow eye or in patients with chronic CSCR. Treatment options include conventional focal laser, photodynamic therapy, and intravitreal drugs like bevacizumab.8, 9, 10 These treatments have had variable outcomes, but not without associated risks and adverse effects. The conventional focal photocoagulation can cause central or paracentral scotomas, contrast sensitivity loss, accidental foveal damage, retinal distortion, or choroidal neovascularization.8, 11 A subfoveal or juxtafoveal leak precludes treatment as well. Although focal laser can potentially seal the leak seen on fluorescein angiography (FFA) and resolve the subretinal fluid, it does not alter the choroidal hyperpermeability and leakage, thus the risk of recurrence remains.12
Newer photocoagulation modalities for retinal diseases include subthreshold micropulse (SM) diode and yellow lasers. They offer the advantage of precise control and spatial confinement of laser lesions to the RPE layer. In micropulse photocoagulation, laser energy is dispensed in a burst or ‘envelope’ of micropulses, instead of a single pulse. This limits the time taken for heat conduction to raise the temperature in the adjacent tissue thereby significantly reducing collateral damage. Repetitive micropulses summate to produce the desirable therapeutic effects with milder retinal irradiances causing lower temperature rise, and hence less or no significant retinal damage.13, 14 Multiple and overlapping spots with no visible clinical end point are delivered to the areas of diseased RPE with the aim of inducing a biological response that promotes the recovery and restoration of the outer blood–retinal barrier and ultimately, the resorption of the subretinal fluid.15 Hence, SM laser unlike conventional laser does not heal with coagulation and scarring, but by stimulation and repair. The ‘ON’ or active laser time and the ‘OFF’ or interpulse time are adjustable by the surgeon, thus controlling the intensity and heat spread.
The SM diode (810 nm) laser has been previously used in the management of CSCR,16, 17, 18 whereas there are only few anecdotal reports (http://bmctoday.net/retinatoday/2010/02/article.asp?f=a-new-treatment-for-chronic-central-serous-retinopathy) on the use of the SM yellow (577 nm) laser so far. The yellow (577 nm) wavelength occurs outside the absorption spectrum of retinal xanthophylls, potentially allowing for treatment close to the fovea.19 The combined absorption by both melanin and oxyhemoglobin of 577 nm causes lesser scatter compared with 532 nm or other yellow wavelengths (561/568 nm). This leads to energy concentration to a smaller volume, allowing use of lower powers and shorter pulse durations. It causes less long-term scarring traditionally caused by older lasers and is as effective as currently used lasers, but with lesser side effects. Microperimetry and fundus autofluorescence (FAF) can further help monitor and quantify the functional and anatomical changes caused by the subthreshold laser burns.
Materials and methods
The study was approved by the institutional review board and was conducted in strict adherence to the tenets of the Declaration of Helsinki. A consecutive series of patients with chronic CSCR (15 eyes of 13 patients) presenting to a tertiary care referral hospital who had undergone treatment with SM yellow laser were retrospectively analyzed. Patients with an idiopathic serous macular detachment of the neurosensory retina on spectral domain optical coherence tomography (SD-OCT) and with a focal or diffuse leak at the level of the RPE on FFA or indocyanine green angiography (ICGA) were included. These patients had been observed for a minimum period of 3 months for spontaneous resolution and then given SD yellow laser treatment. Exclusion criteria included use of exogenous corticosteroids, diabetic retinopathy, uveitis, any hereditary retinal or macular disease, and history of previous retinal treatment for the CSCR.
All patients had undergone a complete ophthalmic examination along with an SD-OCT, FFA, ICGA, and FAF using the Spectralis (Heidelberg Engineering, Germany). The SD-OCT protocol involved horizontal and vertical (100 Automatic Real Time, ART) scans with volume and star-shaped scans (9 ART, 30 degree, 768A scans). The automated segmentation program measured the central retinal thickness. However, we noted that the maximal subretinal fluid height was not always central. Hence, we manually measured the maximal height of the fluid using calipers on the SD-OCT images and used it to assess the treatment outcome.
RPE or retinal changes seen either clinically on SD-OCT or FAF were used to determine the safety of the laser treatment. Microperimetry (CenterVue, Padova, Italy) was done in 8 of 15 eyes at baseline and at follow-up. All patients had given a written informed consent after being explained about the possible benefits and potential risks of SM yellow laser treatment.
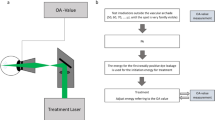
All patients had undergone ICGA before treatment, with both focal leaking points and areas of hyperpermeability forming a guide for laser therapy with 577 nm yellow laser (Supra Scan, Quantel Medical, Cedex, France) delivered through the PDT laser lens (Volk Optical Inc, Mentor, OH, USA). Initially, a continuous wave ‘test’ spot size of 100 μm, 0.2 s exposure time, and using enough power to cause mild retinal whitening was placed superonasally. Using similar settings, but with half the power, a duty cycle of 10% (200 μs on and 1800 μs off) and the micropulse emission mode, multiple overlapping spots were placed over areas of focal and diffuse RPE leak.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Statistical analysis
The raw data were entered on excel sheets (Microsoft Corp., Redmond, WA, USA) and imported to Statistical Package for Social Sciences (SPSS v22, IBM, Armonk, NY, USA) for analysis. The distribution of the data was tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk test. The subretinal fluid measurements were normally distributed, whereas the visual acuity (logMAR) was not. Hence, pre- and post-laser SRF height was analyzed using the paired Student’s t-test and the visual acuity was analyzed using the Wilcoxon Signed-Ranks test.
Results
A total of 15 eyes of 13 patients with a diagnosis of chronic CSCR were recruited and treated with SM yellow laser. Twelve patients were males and one female. All of them had had a history of CSCR of >3 months duration. Their presenting median BCVA was 20/40 (20/20–20/400). Patients were followed up for a minimum period of 4 weeks (range 4–19 weeks) with an average of 8 weeks. The mean age of the patients was 49 years (range 27–63 years). There were eight eyes (53%) with focal leaks and seven (46%) with areas of diffuse leak. Of the patients with focal leaks, one had a ‘smoke stack’ pattern with seven having an ‘inkblot’ pattern (Figure 1). The mean subretinal fluid height was 232 μm (range 85–413 μm). Table 1 summarizes the study data of all patients.
The laser power used in our study ranged from 70 to 200 mW and the average number of burns placed was 264 (72–443). The median BCVA improved by one line from 20/40 to 20/30 (P=0.026). Of the 15 eyes, 2 eyes had 2 lines of improvement (13.3%); 4 eyes had 1 line improvement (27%) and 9 eyes maintained vision (60%).
All patients showed a significant decrease in the subretinal fluid on SD-OCT (Figure 2); the average decrease in fluid height was 79% (P<0.001). Six eyes (40%), three each with focal and diffuse leak, had complete resolution of the subretinal fluid (average 9 weeks (range 1–21 weeks). Four out of 15 eyes (27%) showed a significant (63%; P=0.045%) decrease in fluid height as early as one week. No obvious RPE changes were noted during the follow-up on SD-OCT and FAF. All patients underwent only one session of SM laser. No patients had any other complications during the treatment or follow-up period.
Six of the eight eyes (75%) that underwent microperimetry (Figure 3) showed improved thresholds after laser. The other two eyes of a patient with bilateral involvement and preexisting IO-OS disruption as determined on SD-OCT did not show improved thresholds. However, the vision did improve with reduction in the fluid height.
Discussion
This study demonstrates clinical improvement in visual acuity, following SM yellow laser treatment in CSCR as early as 1 week. Furthermore, statistically significant reduction in subretinal fluid height was noted. Patients were observed for a minimum period of 3 months before treatment and thence offered laser therapy at a stage when spontaneous resolution was unlikely. Hence, it is plausible that the improvement shown in our patients is most likely due to the laser intervention.
There have been previous studies on the use of micropulse (810) diode laser in the treatment of CSCR (Table 2). All studies, including ours, have used visual acuity and changes on SD-OCT as parameters to evaluate response to laser therapy. Lanzetta et al16 reported that subretinal fluid resolved or improved in two-thirds of cases 1 month post laser, and in three-quarters at the end of follow-up. In another study by Ricci et al,17 an OCT scan revealed complete resolution of the serous neuroepithelial detachment in five patients and a marked reduction in two patients. Chen et al18 treated 26 eyes of 25 patients with persistent CSCR with a minimum follow-up of 6 months. Focal leaks without RPE atrophy responded better than those with diffuse leaks with RPE decompensation. At the end of follow-up, the average preoperative foveal thickness was reduced by more than half its original thickness. No response was elicited in 25% of the cases. A gain of visual acuity of 3 lines or more was achieved in 15 eyes (57.7%), and a gain of between 1 and 3 lines was achieved in 6 eyes (23.1%).
Many of our cases showed a diffuse leakage pattern in comparison to other studies, which had a higher proportion of focal leakages. Despite this, a good proportion of eyes responded to treatment and this is probably owing to stimulation of the RPE pump by the low-threshold confluent laser burns placed over the entire diseased RPE.
Microperimetry findings in six patients and post-treatment FAF of six patients ascertain that SM yellow laser is safe, and hence may be considered in acute stages of CSCR or when treating close to the fovea. Microperimetry can also be an important tool in assessing response to therapy, as recovery of retinal sensitivity may precede improvement in visual acuity.
We used lower power settings as compared with the other studies. Lanzetta et al16 used 1–2 W, Koss et al used 800–1740 mW, and Ricci et al17 used 500 mW. We used power in the range of 70–200 mW. This may indicate that yellow laser is better absorbed by RPE and hemoglobin thus producing the desired effects at a much lesser power. Higher pigmentation in our study population of Indian eyes also could be attributable for the less power required. Compared with the other studies with multiple laser sessions, longer follow-ups, and better resolution of SRF, our study reports the early findings of a single sitting of SM yellow laser in CSCR. Although we did not see complete resolution in all cases, our findings are encouraging.
The design of the study was retrospective in nature. Hence, the investigations done and follow-up periods are not standardized or uniform. A study with a larger sample size, with regular and uniform follow-up periods with all patients undergoing FFA, ICGA, FAF, and SD-OCT at every follow-up would be ideal to assess the response to treatment, as well as the safety profile.
We have found SM yellow laser to be an effective option in the management of chronic CSCR. There was a significant anatomical and functional improvement in the treated eyes of our pilot study. As the response was rapid it may be possible to offer this treatment to patients with a significant drop in vision, in those requiring early visual rehabilitation, in those with a massive SRF, or for ‘one-eyed’ patients at the first episode itself. Treatment at an early stage may prevent recurrences and irreversible visual loss. Hence, SM yellow laser can add to the repertoire of treatment options for CSCR.

References
Spaide RF, Goldbaum M, Wong DWK, Tang KC, Iida T . Serous detachment of the retina. Retina 2003; 23: 820–846.
Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D . Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol 1994; 112: 1057–1062.
Gilbert CM, Owens SL, Smith PD, Fine SL . Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 1984; 68: 815–820.
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002; 22: 19–24.
Bujarborua D . Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand 2001; 79: 417–421.
Yannuzzi LA . Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol 2010; 149: 361–363.
Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS . Safety enhanced photodynamic therapy for chronic central serous chorior- etinopathy: one-year results of a prospective study. Retina 2008; 28: 85–93.
Khosla PK, Rana SS, Tewari HK, Azad RU, Talwar D . Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surg Lasers 1997; 28: 693–697.
Ober MD, Yannuzzi LA, Do DV, Spaide RF, Bressler NM, Jampol LM et al. Photodynamic therapy for focal retinal pigment epithelial leaks secondary to central serous chorioretinopathy. Ophthalmology 2005; 112: 2088–2094 30.
Chung YR, Seo EJ, Lew HM, Lee KH . Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye(Lond) 2013; 27 (12): 1339–1346.
Shatz H, Yannuzzi LA, Gitter KA . Subretinal neovascularization following argon laser photocoagulation treatment for central serous chorioretinopathy: complication or misdiagnosis? Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1977; 83: 893–906.
Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF . Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010; 117: 1792–1799.
Mainster MA Laser-tissue interactions: future laser therapies. In: Diabetic Retinopathy: Approaches to a Global Epidemic. Association for Research in Vision and Ophthalmology Summer Research Conference 2010. Natcher Center, National Institutes of Health, Bethesda MD, 31 July 2010.
Mainster MA . Decreasing retinal photocoagulation damage: Principles and techniques. Semin Ophthalmol 1999; 14: 200–209.
Yadav NK, Jayadev C, Rajendran A, Nagpal M . Recent developments in retinal lasers and delivery systems. Indian J Ophthalmol 2014; 62: 50–54.
Lanzetta P, Furlan F, Morgante L, Veritti D, Bandello F . Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol 2008; 18 (6): 934–940.
Ricci F, Missiroli F, Regine F, Grossi M, Dorin G . Indocyanine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2009; 247 (5): 597–607.
Chen SN, Hwang JF, Tseng LF, Lin CJ . Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008; 115 (12): 2229–2234.
Joondeph BC, Joondeph HC, Blair NP . Retinal macroaneurysms treated with the yellow dye laser. Retina 1989; 9: 187–192.
Siviprasad S, Sandhu R, Tandon A, Sayed-Ahmed K, McHugh DA . Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow-up. Clin Experiment Ophthalmol 2007; 35 (7): 640–644.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yadav, N., Jayadev, C., Mohan, A. et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye 29, 258–265 (2015). https://doi.org/10.1038/eye.2014.315
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.315
This article is cited by
-
Half-dose photodynamic therapy versus 577 nm subthreshold pulse laser therapy in treatment-naive patients with central serous chorioretinopathy
BMC Ophthalmology (2024)
-
The effect of nondamaging subthreshold laser therapy in patients with chronic central serous chorioretinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Central serous chorioretinopathy: updates in the pathogenesis, diagnosis and therapeutic strategies
Eye and Vision (2023)
-
Structure and spectral properties of Dy3+ doped CaYAlO4 single crystal
Scientific Reports (2023)
-
Randomized controlled trials in central serous chorioretinopathy: A review
Eye (2023)