Abstract
Aim
The objective of the study was to evaluate the long-term results of allogenic penetrating limbo-keratoplasy. This method allows simultaneous transplantation of a corneal graft and limbal stem cells of the donor by means of eccentric trephination of the donor button.
Method
The data of 192 consecutive cases of allogenic penetrating limbo-keratoplasty from 1995 to 2015 were reviewed. These had been performed exclusively in eyes with complete failure of the limbal stem cells, in combination with deep corneal scarring. Indications were predominantly eye burns, inflammatory conditions, and congenital aniridia. Graft survival and rejection rates were assessed using Kaplan–Meier analysis.
Results
Follow-up averaged 2.1±2.2 years. Median graft survival was 3.4 years in eye burns, 3.9 years in inflammatory disease, and 3.2 years in congenital aniridia. Median survival was 3.9 years in the heterogenous group of other indications.
Conclusion
Allogenic limbo-keratoplasty is a suitable option used to treat patients with bilateral complete failure of the limbal stem cells and deep opacification of the central cornea. The main reasons for graft failure are the loss of graft-limbal stem cell functioning and endothelial graft rejection.
Similar content being viewed by others
Introduction
Limbal stem cells have a key role in maintaining the epithelial layer of the cornea and in corneal wound healing.1 They are the regenerative source of an avascular, stable, and clear corneal epithelium.2 Limbal stem cell failure may be either partial or total (Figure 1). Partial deficiency of the limbal stem cells can usually be treated with selective mechanical debridement in the case of conjunctivalization of the visual axis. This may restore a clear corneal centre in those cases without the need for stem cells. Amniotic membrane transplantation is an additional method.3 Total failure of the limbal stem cells results in severe alteration of the corneal surface, and may lead to complete conjunctivalisation of the cornea, with recurrent ulcerations.4 This results in corneal opacification and visual impairment. There are a multitude of causes for complete limbal stem cell failure. Chemical burns can produce severe damage to the corneal limbus, the corneal stroma, and the anterior ocular segment. Thus, the degree of ischemia and necrosis in limbus, conjunctiva, and sclera may permit the estimation of the damage to the limbal stem cells.5 Aniridia syndrome is associated with several corneal abnormalities, including epithelial erosions and the formation of conjunctival pannus. The abnormality of the limbal stem cell niche due to PAX6 failure is a suspected pathogenesis.6 Chronic inflammatory diseases may also lead to stem cell failure. This is because the inflammation in proximity to the limbal stem cells causes ‘bystander’ damage to the stem cells and their niche. This occurs, for example, in ocular cicatricial pemphigoid,7 blepharitis, atopy, Stevens–Johnson syndrome, and toxic epidermal necrolysis.8 The treatment options for partial and total limbal stem cell failure are summarised in Figure 1.Total limbal stem cell failure can only be cured by the transplantation of viable limbal stem cells. The in vitro expansion of autologous stem cells taken from small limbal biopsies is one therapeutic option.9 This method relies upon the cultivation of autologous stem cells, either in epithelial cell cultures or in fibrin.10 En bloc grafting using the other eye is a less expensive alternative,11 for which large grafts, that cause discomfort and surgical risk in the donor eye, are usually required. This technique can only be applied if the stem cell failure is not bilateral. The advantage of autologous stem cell grafts is that immunological reactions are avoided.9, 10, 11 If both eyes suffer from stem cell failure, the homologous transplantation of the limbal stem cells can be a last resort. Central limbo-keratoplasty was first introduced by Sundmacher et al in 1993.12 The main purpose is to transplant stem cells in their limbal niche together with penetrating keratoplasty from the same donor. This is achieved simply by eccentric trephination of the donor button. The graft may contain up to 40% of limbal tissue13 and is sutured centrally in the recipient bed of the patient, as is the case in conventional penetrating keratoplasty. Advantages of this technique are rapid restoration of the visual acuity owing to the clear donor stroma in the visual axis, and the simultaneous transplantation of the limbal stem cells together with their niche,12, 13, 14 in a single procedure.However, the prognosis of all allogenic techniques is limited due to immunological reactions and conjunctivalisation of the graft.15 The use of mitomycin C (MMC) and amniotic membrane transplantation were later introduced to improve the outcome of limbal stem cell transplantation.16 Subsequently, these additions have been used in allogenic penetrating limbo-keratoplasty.17 Human leukocyte antigen (HLA) matching can be performed additionally to reduce immunogenicity.15, 18 The purpose of this retrospective study was to evaluate the long-term results of the full cohort of allogenic penetrating limbo-keratoplasty in 20 years of experience with this technique, including the ‘learning curve’, and the use of MMC and amniotic membrane transplantation.
Materials and methods
The data of all consecutive cases of allogenic penetrating limbo-keratoplasty from 1995 to 2015 were analysed. Ethics committee approval was obtained at Albert-Ludwigs-University Freiburg (88/12). The patient information was anonymised and de-identified prior to analysis. The data from 1995 to 2003 pertained to patients from the Department of Ophthalmology, University Hospital, Düsseldorf, Germany, and those from 2003 to 2015 to patients from the Eye Center at the Medical Center, University of Freiburg, Germany.
Surgical technique
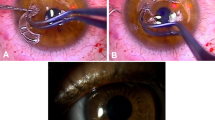
The technique for limbo-keratoplasty has been described by Sundmacher et al12 The graft tissue is eccentrically trephined in order to obtain limbal tissue for transplantation. The recipient cornea is removed with a trephine, usually 0.25 mm smaller than that used for the preparation of the graft. Suturing of the graft is performed with a double-running Hoffmann suture (10-0 nylon). A concentration of 0.02% was intraoperatively administered for 2 minutes for MMC use. Thereafter, intensive rinsing of the cornea was performed before trephination of the host cornea. The amniotic membrane was additionally grafted to cover the grafted limbal tissue. After transplantation, a bandage contact lens was placed on the eye.
Postoperative treatment
Supportive treatment included the administration of topical and systemic steroids, as well as systemic immunosuppression with mycophenolate mofetil or cyclosporine A.
Topical steroids were administered five times daily for the first month after keratoplasty. The number of eye drops was reduced over a period of 5 months. Thereafter, topical steroids were administered at least once a day permanently unless side-effects occurred, which justified termination of the treatment.
Cyclosporin A was administered orally. The dosage was adapted according to the patient’s plasma levels. Mycophenolate mofetil was administered orally at 1 g twice daily. During immunosuppression, the patients underwent a regular examination and blood testing at their general practitioner in order to timeously diagnose the side effects of the medication. Unless serious side effects occurred, immunosuppression was maintained for at least 6 months.
Each transplantation was classified into one of four groups, namely:
-
Eye burns: This included patients with acid and alkali eye burns with consecutive damage to the limbal stem cells (Figure 2a).
-
The congenital aniridia group: This comprised patients with iris dysgenesis and limbal stem cell failure (Figure 2b).
-
The inflammatory group: This comprised patients with limbal stem cell failure due to Stevens–Johnson syndrome, Lyell’s syndrome, or ocular citatrical pemphigoid.
-
Other indications: This group included patients with any other disease with consecutive complete limbal stem cell failure, that is, corneal ulcers, blepharokeratoconjunctivitis and various corneal dystrophies.
Any other ocular surface pathology, such as symblepharon or lid margin, were treated before the limbo-keratoplasty. The number of HLA mismatches and the postoperative use of systemic immunosuppressive agents, either with mycophenolate mofetil, cyclosporine A, or a combination of both, was also recorded. The recurrence-free survival of the graft and rejection-free graft survival were estimated using Kaplan–Meier analysis. The grafted limbus and the donor endothelium are capable of being rejected independently. Therefore, the immune reaction to the graft endothelium and rejection of the graft limbus were assessed separately. The endothelial immune reaction was diagnosed when the graft showed endothelial precipitates and/or corneal oedema that was otherwise unexplained. Limbal rejection was deemed to have occurred when a hyperaemic reaction occurred in the grafted tissue together with an endothelial reaction, or when there were new signs of limbal failure in an otherwise regular graft. Improvement was also determined in best corrected visual acuity (BCVA) using Kaplan–Meier analysis because there was not a consistent single postoperative point in time during which visual acuity data was available for all patients. We opted to achieve spectacle-corrected acuity of at least 20/200 as an end point because this is commonly considered to be an important milestone in quality-of-life research.19 The demographic data are summarised in Table 1.
Results
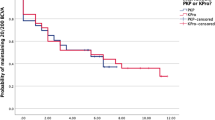
One hundred and ninety-two cases of allogenic limbo-keratoplasty were assessed. Overall median graft survival was 3.43 years. Diagnoses included eye burns (n=78), congenital aniridia (n=18), inflammatory disease (n=58), and other indications (n=38). The follow-up duration was an average of 2.1±2.2 years. Median graft survival was 3.4 years for eye burns, 3.2 years for congenital aniridia, 3.9 years for inflammatory disease, and 3.9 years for other indications (Kaplan–Meier analysis; Figure 3a).
(a) Kaplan–Meier estimation of graft survival in homologous limbo-keratoplasty for complete limbal stem cell failure. Eye burns included acid and alkali burns with consecutive damage to the limbal stem cells; congenital aniridia included eyes with incomplete formation of the iris and additional complete limbal stem cell failure, and inflammatory diseases limbal stem cell failure due to Stevens–Johnson syndrome, Lyell’s syndrome, or ocular citatrical pemphigoid. Other indications were diseases with consecutive complete limbal stem cell failure. (b) Kaplan–Meier estimation of graft survival with regard to endothelial rejection. Eye burns included acid and alkali burns with consecutive damage to the limbal stem cells; congenital aniridia included eyes with incomplete formation of the iris and additional complete limbal stem cell failure, and inflammatory diseases limbal stem cell failure due to Stevens–Johnson syndrome, Lyell’s syndrome, or ocular citatrical pemphigoid. Other indications were diseases with consecutive complete limbal stem cell failure.
Rejection-free graft survival was assessed after 3 years. Rejection-free graft survival of 43% was observed for eye burns, 25% for congenital aniridia, 30% for inflammatory disease, and 68% for all other indications (Kaplan–Meier analysis; Figure 3b). At least one rejection of the graft was reported during the follow-up for 25% of the grafts (Kaplan–Meier analysis). HLA typing was performed in 114 cases. A close match (0–1 mismatch) was achieved in 28 patients and there were 2 mismatches in 48 patients. HLA typing was not available for the rest. This was especially true for patients reviewed between 1994 and 1996. Thereafter, considerable efforts were made to ensure HLA matching in every patient. Poor matchability, due to rare or homozygous haplotypes, was the reason for failed HLA matching in some.20
Median graft survival was 3.9 years in the group with matched grafts, 2.6 years for those with unmatched grafts, and 2.9 years for patients with untyped grafts. This effect was not statistically significant (P=0.880). Systemic immunosuppression, achieved with either the administration of mycophenolate mofetil or cyclosporine A, is the standard treatment that we use after limbo-keratoplasty. Initially, the patients received cyclosporine A in the study. Mycophenolate mofetil was prescribed later on as soon as it became commercially available. Mycophenolate mofetil and/or cyclosporine A was administered for 92% of the transplantations. A combination of the two was prescribed in 41%. Systemic immunosuppressive agents could not be administered to patients with contraindications, for example, those with tumours or infections such as tuberculosis. Topical steroids were administered in all cases for as long as possible. Median graft survival in patients given cyclosporine A was 4.1 years, 3.9 years in those given mycophenolate mofetil, 2.6 years in those given both, and 2.4 years in patients to whom immunosuppressive agents could not be administered. Statistical significance was not demonstrated by these results (P=0.057).
Nine patients received additional intraoperative treatment with MMC and amniotic membrane transplantation. Median graft survival for those patients was 2 years, compared with 3.4 years for the rest.
The BCVA data were available for analysis of the Freiburg subgroup exclusively. Also, BCVA above 20/200 was recorded for the patients with other indications after 22 months, and the same for patients with eye burns after 47 months. Only 17% of the group of congenital aniridia patients achieved BCVA of above 20/200, while 100% of those with inflammatory disease did so after 45 months. After the initial gain, visual acuity declined during follow-up in all groups due to graft failure (Figure 4).
Discussion
Allogenic limbo-keratoplasty is a surgical option for patients in whom any form of corneal surgery, other than keratoprothesis, is unsuitable.
This is especially the case after bilateral total failure of the limbal stem cells in combination with severe deep corneal opacification. The purpose of our study was to evaluate the long-term outcomes of limbo-keratoplasty in the major indication groups. To the best of our knowledge, this was the largest and most long-term dataset analysed to date. A major finding was the reduced long-term prognosis with respect to clear graft survival. Graft failure can be caused either by graft rejection or failure of the limbal stem cells inside the limbal portion of the graft. It was demonstrated in our study that overall graft survival could not be explained by endothelial graft rejection alone. It is most likely that non-endothelial failure is due to degeneration or inflammation of the grafted limbal stem cells in some transplants. The limbal tissue is both prone to rejection by the host and failure of the vascular connection to the limbal vascular arcade. A combination of limbo-keratoplasty, amniotic membrane transplantation, and the intraoperative use of MMC has been described with respect to complete bilateral failure of the limbal stem cells.17, 21
Protection of the grafted limbal tissue from epithelialisation by the conjunctiva is the main advantage of this technique, which is achieved by inhibition of the proliferation of the conjunctival stem cells. The use of MMC and amniotic membrane transplantation did not prolong graft survival in our study, in comparison with that in the group receiving conventional limbo keratoplasty. This may be attributed to a selection bias because this technique was used in the most severe cases. In addition, the use of MMC and amniotic membrane transplantation may not prevent rejection of the limbus or the endothelium. They do not prevent graft rejection.
Without immunosuppression, the failure rate of allogenic limbal stem cell transplantation is high due to graft rejection.12 The use of matched grafts was proposed to minimise graft failure.18 The longest median graft survival was demonstrated in patients with matched grafts in our study. However, this result was not statistically significant. There was a tendency for longer median graft survival with the administration of systemic immunosuppressive agents, mycophenolate mofetil and cyclosporine A. Surprisingly, a combination of both immunosuppressant agents seemed to be less effective than mycophenolate mofetil or cyclosporine A alone. Again, this may be owing to a selection bias. In particular, patients with a very high risk of graft rejection were treated with a combination of both immunosuppresive agents. The survival of the stem cells is crucial to survival and function of the graft. Survival of the donor cells can be seen by the lack of conjunctivalisation during the slit lamp examination and by the degree of visual acuity, and has been determined with the use of genetic analysis of the epithelium after limbo-keratoplasty.22 The visual acuity results were good compared with the preoperative visual acuity in our study, although the optical quality of the graft may have been reduced by an irregular astigmatism due to eccentric trephination. However, this disadvantage was negligible, since most patients have low visual acuity and the astigmatism can be corrected with a rigid contact lens.
Fifty percent of our patients obtained BCVA of at least 20/200. This milestone was reached earlier or later depending on the original problem. The most rapid improvement (9 months) was recorded in the inflammatory group of patients. The worst prognosis with respect to visual acuity was associated with the group of patients with congenital aniridia. This may be attributed to ocular comorbidities, such as macular hypoplasia, which limit vision. Interestingly, the lowest rate of endothelial graft failure was also seen in congenital aniridia patients. This may be owing to younger patient age and a larger pool of remaining host endothelial cells.23 Over time, visual acuity declines in all groups owing to the high rate of graft failures (Figure 4). BCVA and the overall graft survival of limbo-keratoplasty compare favourably with the Boston keratoprosthesis method. Vision better than 20/200 in 50% of 150 patients after 3–7 years was demonstrated with the latter.24 A significant number of patients with loss of vision postoperatively has been indicated.25 Secondary glaucoma is reported to be the main reason for visual loss.26 The rate of secondary glaucoma has been reported as being as high as 64%.27 The use of glaucoma drainage devices in such patients may result in associated complications, including visual loss.28 Limbo-keratoplasty seems to be a promising option given the outcomes in our study. However, more data are required in order for a sound analysis to be conducted.
Other treatment options such as the ex vivo expansion of the limbal stem cells on amniotic membranes or fibrin have been described. However, not many long-term data are available.29, 30 Furthermore, it is unclear whether complete deficiency was applicable to all the patients. The technique of a lamellar central limbo-keratoplasty has also been described.14 Less invasive techniques are possible for partial limbal stem cell failure3 (Figure 1).
Limitations
There were several limitations to our study, especially with respect to its retrospective design. In addition, not all data were available for the initial Düsseldorf patients. Improvement over time was demonstrated with the use of supportive medical therapy in conjunction with the introduction of systemic immunosuppressive agents, and the intraoperative use of MMC and amniotic membrane transplantation. Therefore, the treatment differed in patients with the same disease. It is likely that more severe cases were primarily treated with immunosuppressive agents, MMC, and amniotic membrane transplantation thus leading to limited success with respect to our statistical analysis. The follow-up of the patients also differed widely. This may have biased our results as some of the patients with graft failure may not have returned.
Conclusion
A beneficial therapeutic option is provided by penetrating limbo-keratoplasty for patients with complete limbal stem cell failure. Long-term evidence of the efficacy of penetrating limbo-keratoplasty was reported in this article. The survival of the graft depends on that of the transplanted limbal stem cells. Systemic immunosuppressive agents and HLA matching should be utilised to prolong graft survival.

References
Tseng SC . Concept and application of limbal stem cells. Eye 1989; 3 (Pt 2): 141–157.
Yeung A, Schlötzer-Schrehardt U, Kulkarni B, Tint NL, Hopkinson A, Dua HS . Limbal epithelial crypt: a model for corneal epithelial maintenance and novel limbal regional variations. Arch Ophthalmol 2008; 126 (5): 665–669.
Dua HS, Saini JS, Azuara-Blanco A, Gupta P . Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 2000; 48 (2): 83–92.
Chen JJ, Tseng SC . Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci 1991; 32 (8): 2219–2233.
Wagoner MD . Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol 1997; 41 (4): 275–313.
Ramaesh K, Ramaesh T, Dutton GN, Dhillon B . Evolving concepts on the pathogenic mechanisms of aniridia related keratopathy. Int J Biochem Cell Biol 2005; 37 (3): 547–557.
Samson CM, Nduaguba C, Baltatzis S, Foster CS . Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology 2002; 109 (5): 862–868.
Vera LS, Gueudry J, Delcampe A, Roujeau J-C, Brasseur G, Muraine M . In vivo confocal microscopic evaluation of corneal changes in chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea 2009; 28 (4): 401–407.
Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M . Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997; 349 (9057): 990–993.
Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G . Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010; 363 (2): 147–155.
Tan DT, Ficker LA, Buckley RJ . Limbal transplantation. Ophthalmology 1996; 103 (1): 29–36.
Sundmacher R, Reinhard T, Althaus C . Homologous central limbo-keratoplasty in limbus stem cell damage. retrospective study of 3 years’ experience. Ophthalmologe 1997; 94 (12): 897–901.
Sundmacher R, Reinhard T . Central corneolimbal transplantation under systemic ciclosporin a cover for severe limbal stem cell insufficiency. Graefes Arch Clin Exp Ophthalmol 1996; 234 (Suppl 1): S122–S125.
Sundmacher R, Reinhard T . Homologous lamellar central limbokeratoplasty in severe limbal stem cell deficiency. Klin Monbl Augenheilkd 1998; 213 (4): 254–255.
Reinhard T, Spelsberg H, Henke L, Kontopoulos T, Enczmann J, Wernet P et al. Long-term results of allogeneic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology 2004; 111 (4): 775–782.
Liang L, Li W, Ling S, Sheha H, Qiu W, Li C et al. Amniotic membrane extraction solution for ocular chemical burns. Clin Exp Ophthalmol 2009; 37 (9): 855–863.
Eberwein P, Böhringer D, Schwartzkopff J, Birnbaum F, Reinhard T . Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin C, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology 2012; 119 (5): 930–937.
Reinhard T, Sundmacher R, Spelsberg H, Althaus C . Homologous penetrating central limbo-keratoplasty (HPCLK) in bilateral limbal stem cell insufficiency. Acta Ophthalmol Scand 1999; 77 (6): 663–667.
Brown MM, Brown GC, Sharma S, Busbee B . Quality of life associated with visual loss: a time tradeoff utility analysis comparison with medical health states. Ophthalmology 2003; 110 (6): 1076–1081.
Böhringer D, Reinhard T, Böhringer S, Enczmann J, Godehard E, Sundmacher R . Predicting time on the waiting list for HLA matched corneal grafts. Tissue Antigens 2002; 59 (5): 407–411.
Birnbaum F, Böhringer D, Sokolovska Y, Sundmacher R, Reinhard T . Immunosuppression with cyclosporine A and mycophenolate mofetil after penetrating high-risk keratoplasty: a retrospective study. Transplantation 2005; 79 (8): 964–968.
Spelsberg H, Reinhard T, Henke L, Berschick P, Sundmacher R . Penetrating limbo-keratoplasty for granular and lattice corneal dystrophy: survival of donor limbal stem cells and intermediate-term clinical results. Ophthalmology 2004; 111 (8): 1528–1533.
Böhringer D, Böhringer S, Poxleitner K, Birnbaum F, Schwartzkopff J, Maier P et al. Long-term graft survival in penetrating keratoplasty: the biexponential model of chronic endothelial cell loss revisited. Cornea 2010; 29 (10): 1113–1117.
Srikumaran D, Munoz B, Aldave AJ, Aquavella JV, Hannush SB, Schultze R et al. Long-term outcomes of boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology 2014; 121 (11): 2159–2164.
Greiner MA, Li JY, Mannis MJ . Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology 2011; 118 (8): 1543–1550.
Ahmad S, Akpek EK, Gehlbach PL, Dunlap K, Ramulu PY . Predictors of visual outcomes following Boston type 1 keratoprosthesis implantation. Am J Ophthalmol 2015; 159 (4): 739–747.
Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI . Boston keratoprosthesis: outcomes and complications: a report by the American Academy of Ophthalmology. Ophthalmology 2015; 122 (7): 1504–1511.
Li JY, Greiner MA, Brandt JD, Lim MC, Mannis MJ . Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol 2011; 152 (2): 209–218.
Eberwein DP, Reinhard T . Perspektiven und aktueller stand der Limbusstammzell transplantation. Ophthalmologe 2011; 108 (9): 840–845.
Tsai RJ-F, Tsai RY-N . From stem cell niche environments to engineering of corneal epithelium tissue. Jpn J Ophthalmol 2014; 58 (2): 111–119.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lang, S., Böhringer, D., Geerling, G. et al. Long-term results of allogenic penetrating limbo-keratoplasty: 20 years of experience. Eye 31, 372–378 (2017). https://doi.org/10.1038/eye.2016.217
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.217
This article is cited by
-
Therapie des okulären Schleimhautpemphigoids
Die Ophthalmologie (2023)
-
Influence of graft vascularization on graft survival following homologous limbo-keratoplasty
International Ophthalmology (2022)
-
An attempt to optimize the outcome of penetrating keratoplasty in congenital aniridia-associated keratopathy (AAK)
International Ophthalmology (2021)
-
Nekrotisierende Skleritis nach Akanthamöbenkeratitis
Der Ophthalmologe (2021)
-
Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review
Ophthalmology and Therapy (2020)